Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Anubha Bajaj*
Received: May 05, 2023; Published: May 18, 2023
*Corresponding author: Anubha Bajaj, Consultant Histopathologist, A.B. Diagnostics, A-1, Ring Road, Rajouri Garden, India
DOI: 10.26717/BJSTR.2023.50.007961
Abbreviations: APC: Adenomatous Polyposis Coli; PAS: Periodic acid Schiff’s; CEA: Carcinoembryonic Antigen; AFP: Alfa Fetoprotein; ACTH: Adrenocorticotropic Hormone; CT: Computerized Tomography; MRI: Magnetic Resonance Imaging; FNAC: Fine Needle Aspiration Cytology
Pancreatoblastoma is an exceptionally discerned, malignant pancreatic tumour configured of multiple pancreatic cellular lines of differentiation and an aggressive clinical course. Tumour cells demonstrate absence of chromosome 11p along with alterations within adenomatous polyposis coli (APC) gene or beta catenin signalling pathway. Generally, pancreatoblastoma displays minimally two pancreatic cellular lines of differentiation and is composed of acinar, ductal or neuroendocrine elements. Squamoid cell nests emerge as a definitive, diagnostic feature. Additionally designated as infantile pancreatic carcinoma or pancreatic carcinoma of childhood, pancreatoblastoma may be appropriately subjected to complete surgical resection. Comprehensive surgical eradication of the neoplasm represents as a singular crucial prognostic indicator of disease outcome [1,2]. In contrast to adult population, pancreatoblastoma frequently arises within children wherein tumefaction is a commonly encountered pancreatic neoplasm incriminating children < 10 years. Median age of disease emergence is 4 years to 5 years. Mean age of disease representation within adults is 41 years. An equivalent gender predilection is enunciated [1,2]. An estimated 50% of pancreatoblastomas occur within head of pancreas followed in frequency by neoplasms confined to tail of pancreas or body of pancreas. Exceptionally, ampulla of Vater may be incriminated [2,3]. Of obscure pathogenesis, pancreatoblastoma commonly demonstrates loss of chromosome 11p. Tumour is posited to exhibit genomic mutations within Adenomatous Polyposis Coli (APC) gene or beta catenin signalling pathway. Of obscure aetiology, few instances of pancreatoblastoma appear associated with Beckwith-Wiedemann syndrome or familial adenomatous polyposis [2,3].
Pancreatoblastoma manifests with nonspecific clinical symptoms. Abdominal pain is commonly encountered. Frequently, tumefaction is advanced upon initial clinical representation and accompanied by localized tumour extension or distant metastases. Generally, tumour metastasis occurs within hepatic parenchyma, regional lymph nodes or pulmonary parenchyma. Pancreatoblastoma is commonly associated with Beckwith-Wiedemann syndrome or familial adenomatous polyposis [2,3]. Upon gross examination, an enlarged, lobulated, fleshy tumour mass with an average diameter of 10 centimetres is discerned. Foci of cystic degeneration or soft areas of tumour necrosis are encountered. Also, Beckwith-Wiedemann syndrome is associated with predominantly cystic neoplasms. Cytological examination exhibits a loosely cohesive tumour composed of miniature, blast-like tumour cells imbued with granular cytoplasm, fine nuclear chromatin and distinctive nucleoli. Focal cellular and nuclear atypia is exemplified [2,3]. Upon cytology, tumour may simulate acinar cell carcinoma pancreas. Squamoid cell nests appear as well defined clusters, whorls, cellular accumulates or syncytial aggregates. Neoplastic cells may be appropriately exemplified upon examination of cytological cell blocks. Upon microscopy, tumour is composed of multi-lineage pancreatic components, reminiscent of embryonic pancreas. Distinct solid, geographic, hyper-cellular neoplastic lobules appear demarcated by bands of fibrous tissue [2,3].
Focal acinar differentiation is predominant and tumefaction may simulate acinar cell carcinoma. Tumour zones of acinar cells simulate normal acini with articulation of miniature lumens and accumulated intraluminal secretion. Foci of solid, trabecular or pseudo-glandular tumour configurations are encountered [3,4]. Cellular cytology is characteristically bland. Tumour cells appear incorporated with granular, eosinophilic or amphophilic cytoplasm, minimally atypical nuclei and miniature nucleoli. Besides, aggregates of plump spindle shaped cells permeated with abundant eosinophilic or clear cytoplasm may articulate neoplastic whorls or nests. Squamoid cellular nests are comprehensively observed and appear as a distinctive feature. Foci of keratinization may appear [3,4]. Focal neuroendocrine component is observed in > 50% tumours. Neuroendocrine cells are diffusely commingled with acinar component or manifest with focal organoid or trabecular configuration and may be discernible with cogent immunohistochemistry [3,4]. Ductal component appears as a focal, minimally glandular formation which may be appropriately discerned with mucicarmine stain. Additionally, neoplasm exhibits a primitive component with configuration of solid sheets of immature, miniature spherical cells. The stroma is variably cellular and occasionally exhibits heterologous elements as bone or cartilage [3,4]. Upon ultrastructural examination, tumours depicting zymogen granules of magnitude 400 nanometres to 800 nanometres exhibit foci of acinar differentiation. Neoplasms with neuroendocrine differentiation delineate granules of magnitude 125 nanometres or 250 nanometres Besides, mucigen granules of magnitude 500 nanometres to 900 nanometres may be encountered [3,4] (Figures 1 & 2).
TNM staging of pancreatoblastoma is identical to TNM classification of carcinoma of exocrine pancreas [3,4]. Pancreatoblastoma is immune reactive to pancytokeratin, beta catenin, CK5, epithelial membrane antigen (EMA), trypsin, chymotrypsin, lipase or BCL10. Tumour can be stained with periodic acid Schiff’s (PAS) stain with diastase resistance. Ductal immune markers as Carcinoembryonic Antigen (CEA), B72.3, DUPAN-2, CK19 or mucicarmine and neuroendocrine markers as synaptophysin, chromogranin or INSM1 appear reactive. Instances with elevated serum Alfa Fetoprotein (AFP) appear immune reactive to AFP [4,5]. Pancreatoblastoma is immune non reactive to diverse germ cell markers and p53. Exceptionally, absence of DPC4 expression is encountered. Pancreatoblastoma requires segregation from neoplasms such as acinar cell carcinoma, neuroendocrine carcinoma, solid pseudo-papillary neoplasm, undifferentiated carcinoma or anaplastic carcinoma [4,5]. An estimated 50% of incriminated paediatric subjects demonstrate elevated levels of carcinoembryonic antigen (CEA) and alpha fetoprotein (AFP). Around two thirds (66%) of incriminated children enunciate an alpha fetoprotein (AFP) > 1000 micrograms/ litre. However, neoplasms occurring in adults exhibit a variable concurrence with aforesaid biomarkers. Certain neoplasms secrete Adrenocorticotropic Hormone (ACTH) with consequent emergence of Cushing’s syndrome [4,5]. Upon radiography, a solid, irregular lesion is commonly encountered. However, lesion can be cystic or degenerative. Tumefaction may demonstrate extraneous expansion. Computerized tomography ( CT) and magnetic resonance imaging (MRI) can be optimally adopted to discern pancreatoblastoma. Cogent detection upon fine needle aspiration cytology (FNAC) may be challenging due to diversity of expressed cellular lineages [4,5]. Comprehensive surgical resection of the neoplasm is an optimal, recommended mode of therapy which demonstrates crucial prognostic outcomes. Besides, chemotherapy or radiotherapy may be employed to ensure complete eradication of the neoplasm [4,5]. Prognostic outcomes are preponderantly contingent to resectability of the neoplasm. Resectable tumours exhibit a 5 year survival of ~ 65% whereas non resectable tumours exemplify a 5 year survival of 0%. Paediatric subjects exemplify a well encapsulated tumour which is devoid of metastases. Neoplasms occurring in adults or emergence of distant metastasis is associated with inferior prognostic outcomes [4-7].
Figure 1 Pancreatoblastoma depicting aggregates of tumour cells imbued with granular, eosinophilic cytoplasm, mild nuclear atypia and conspicuous nucleoli. Foci of acinar cells, squamoid cell clusters and neuroendocrine cells appear intermingled within a spindle shaped cellular stroma [6].
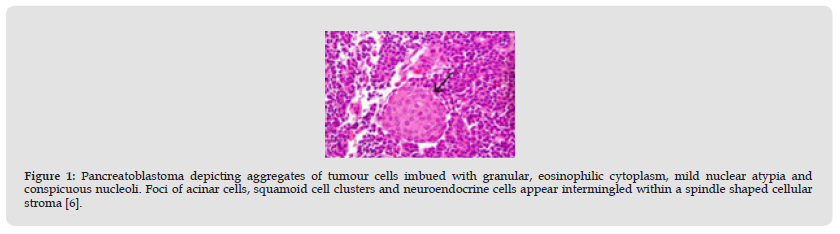
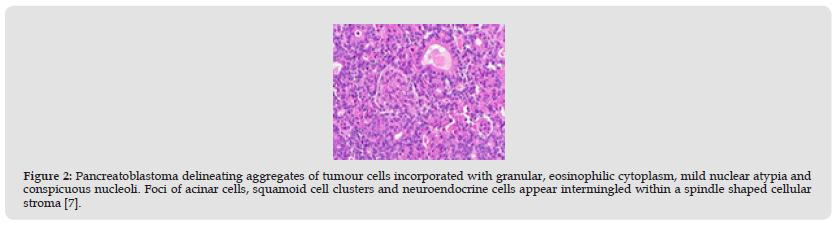
Figure 2 Pancreatoblastoma delineating aggregates of tumour cells incorporated with granular, eosinophilic cytoplasm, mild nuclear atypia and conspicuous nucleoli. Foci of acinar cells, squamoid cell clusters and neuroendocrine cells appear intermingled within a spindle shaped cellular stroma [7].