Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Bhavna Kumari, Sachin Kumar, Prasad Thota*, Manoj Kumar Pandey, Rajeev Singh Raghuvanshi and Anil Kumar Teotia
Received: May 04, 2023; Published: May 18, 2023
*Corresponding author: Prasad Thota, Microbiology Division, Indian Pharmacopoeia Commission, Ministry of Health & Family Welfare, Govt. of India Sector-23, Raj Nagar, Ghaziabad-201 002, U.P., India
DOI: 10.26717/BJSTR.2023.50.007959
In India the prevalence of acidity due to poor eating habit is high in Indian population. Unfortunately, the use of antacids is common which is widely available over the counter (OTC) drug in all pharmaceutical outlets. The primary healing advantage of antacids is the rapid onset of action, which provides immediate ease of gastric discomfort. Most antacids are water-based preparations, and the microbial bioburden of pharmaceuticals has long been a source of concern for pharmaceutical manufacturers around the world among varied pharmaceutical preparations. The presence of contaminants can cause physicochemical alterations in pharmaceutical drugs. The existence of microbial burden is analytically permissible in nonsterile pharmaceuticals regardless of dosage form; though, they should meet the suitable microbiological quality benchmark and meet the acceptance limit mentioned in recent edition of Indian pharmacopoeia (IP) 2022. The objective of this study was to enumerate the total aerobic microbial count (TAMC) and total yeast and mold count (TYMC) in antacids and check the presence and absence of specified pathogens. A total of twenty antacids samples in aqueous and non-aqueous form of preparation were tested. Our study’s findings showed that microbial contamination varied among the pharmaceutical preparations, with the highest microbial contamination in aqueous preparations and the lowest in non-aqueous preparations. The isolated microbial contaminations were of human flora types, i.e., gram positive or air borne fungi. This study leads us to the conclusion that microbiological quality control is crucial for both the quality of the product and risk reduction for the end user.
Keywords: Antacids; Microbiological Quality; Quality Control; Microbial Contamination; Non-Sterile Pharmaceuticals
Abbreviations: USFDA: United States Food and Drug Administration; OTC: Over the Counter; GERD: Gastroesophageal Reflux Disease; NSP: Non Sterile Pharmaceuticals; USP: United States Pharmacopoeia; EP: European Pharmacopoeia; IP: Indian Pharmacopoeia; TAMC: Total Aerobic Microbial Count; TYMC: Total Yeast and Mold Count; CFU: Colony Forming Unit; HEPA: High Efficiency Particulate Air; LAF: Laminar Air Flow; GPT: Growth Promotion Test; SCDM: Soyabean Casein Digest Medium; SCDA: Soyabean Casein Digest Agar; SDA: Sabouraud Dextrose Agar; NSQ: Not of Standard Quality; GMP: Good Manufacturing Practices; GLP: Good Laboratory Practices
A pharmaceutical drug is a chemical entity, when administered alters the functioning of living body or produce biological effect. The drugs are intended to be used for diagnosis, treatment, mitigation, or prevention of a disease and at the same time relieving the discomfort to the user [1]. Antacids are the most widely used self-prescribed medications because poor eating habits, spicy food, and consuming large amounts of carbonated beverages disturb the pH balance and natural flora of the stomach, causing both bloating and acidity [2]. As per the United States Food and Drug Administration (USFDA), antacids are categorized as pharmaceutical drugs that counteract stomach acids and are widely accessible over the counter (OTC) drugs in all the pharmacy outlets [3]. Antacids have been in use to treat gastric disorders and gained popularity in the early nineteenth century [4]. The primary healing advantage of using antacids is their rapid onset of action, which provides immediate ease of gastric discomfort. The normal gastric pH of the stomach lies in the range of 1.5 to 3.5 [1]. Excessive secretion of these gastric acids can inflame the stomach, cause ulcers and other gastroesophageal reflux disease (GERD). According to a prevalence survey carried out in 2016, the prevalence of GERD was determined to be 22.2% in southern India alone [3]. Antacids are generally categorized under two groups: those that work by chemical neutralization of gastric acid; and those that work by adsorption of the acid. The majority of antacids used in clinical settings is non-absorbable and function by raising pH levels, reducing acidity, or preventing the gastric cells ability to secrete acid. Unfortunately, due to their widespread use, these drugs are frequently recommended for mild instances of indigestion or heartburn. Antacids are generally used in the form of tablets or in suspensions [5]. After analgesics and anti-allergic, antacids are ranked among the third most sold OTC drugs making these drugs highly vulnerable for its misuse [3]. Generally, pharmaceutical products are typically categorized as either sterile or non-sterile. Sterile pharmaceuticals entail the production of medicines in an area free from microorganisms, which includes the manufacturing of parenteral preparations, whereas non-sterile pharmaceuticals (NSP), which also need a clean area but allow the presence of non-objectionable microorganisms within allowable limits. The recommended microbial load in the NSP should be rigorously maintained following specifications given in the United States Pharmacopoeia (USP 2022), European Pharmacopoeia (EP 11.0) or Indian Pharmacopoeia (IP 2022) [6-8]. NSP is available in topical dosage forms, including creams, gels, and ointments, as well as oral dosage forms, such as tablets, capsules, syrups, suspensions, and emulsions [9]. The existence of microbial load is theoretically permissible in non-sterile pharmaceuticals regardless of dosage form; however, they must meet the ideal microbiological quality standards and normal levels following specifications provided in various pharmacopoeias. The microbial limit test for total aerobic microbial count (TAMC) and total yeast and mould count (TYMC) should fall within ideal microbiological limits and be free from certain pathogens such as Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Salmonella sp., Shigella sp., Clostridium sp., Bile-tolerant gram-negative bacteria, and Candida albicans, as specified in Indian Pharmacopoeia [6]. The acceptance criteria for microbiological quality of nonsterile dosage form of aqueous and non-aqueous preparation for oral use is mentioned in (Table 1). Non sterile oral drugs show the presence of various types of microbes which may result in the physicochemical deterioration of both active and inactive ingredients of the pharmaceutical product [10]. Further, possibility of presence of microbes in the final products may have hazardous health effects to the end users. The level of microbial contamination in the NSP depends on the various factors viz. oxygen content, amount of microbial existence and the availability of nutrients [11]. Microbial evaluation of the pharmaceutical products is one of the major quality control parameters. Such microbial evaluation is based on the qualitative and quantitative tests meant for the determination of the microbial content in the pharmaceutical drugs [12]. Several studies have been conducted on the microbial evaluation in antacids from different countries viz. Sudan, Nigeria, Tanzania etc [10-13]. Unfortunately, there is scarcity in such studies on antacids from India. Hence, the present study was framed to enumerate the bacterial and fungal counts in antacids purchased from the local markets of Ghaziabad, India and to check the presence of specified pathogens using the conventional pharmaceutical microbial testing method as described in Indian Pharmacopoeia.
Collection of Antacids Drugs
Antacids samples belonging to various manufacturers were collected from different rural areas of Ghaziabad districts, Uttar Pradesh, India. As antacids are OTC medicines, it is easily collected from many pharmacy and Government outlets. A total of 20 antacids drugs were collected, of them 17 were in oral suspensions forms and the remaining were in tablets forms.
Criteria for Physical Examination of Drugs
In order to determine the physical quality and originality of the product, the physical examination of the drugs is done based on appearance, odor, manufacturer’s name, the date of manufacturing and expiration. Additionally, the packaging material was checked for cracks and leakage.
Controlled Area
To prevent items and test preparations from environmental contamination, all sample preparation and analysis were done in a controlled environment under horizontal laminar air flow. The laminar air flow (LAF) hood used had 99.99% efficiency to filter microorganisms and particulates with a 0.3μ high efficiency particulate air (HEPA) filter.
Instruments Used
The instruments used during the study were top pan balance (Aczet Pvt. Ltd., India), autoclave (P.L. Scientific Instruments, India), laminar air flow (Toshiba, India), BOD incubator (M.K. Scientific Instruments, India), colony counter (Mac, India), water bath (Instech System, India), microscope (Olympus, Japan), pH meter (Mettler Toledo, United States), and hot air oven (Tanco, India).
Media preparation and growth promotion test (GPT)
The dehydrated media required in this study were procured from Himedia Laboratories Private Limited, Mumbai, India. The culture media used during the analysis are soyabean casein digest medium (SCDM) as a diluent for the pre-treatment of the sample, soyabean casein digest agar (SCDA) for TAMC, sabouraud dextrose agar (SDA) for TYMC, MacConkey broth for pre-enrichment of E. coli, and MacConkey agar as a selective media for E. coli. The prepared media were sterilized in an autoclave at 121°C and 15 psi for 20 min and checked for growth promotion, indicative and inhibitory test following the Indian Pharmacopoeia [7].
Negative Control
Use the diluent soyabean casein digest medium as a negative control in place of the test organisms (inoculum) to confirm the test conditions. There should not be any growth of microorganisms in this control.
Preparation of Samples
A 1:10 dilution of test sample was prepared aseptically using 10 gm (in case of tablets) or 10 mL (in case of suspension) sample in sterilized SCDM as per the procedure mentioned in the general chapter microbial contamination in non-sterile products of Indian Pharmacopoeia (IP 2022). Further serially dilution of the sample was done using the same diluent as mentioned above for the enumeration of TAMC and TYMC.
Microbial Enumeration Test
The conventional pour plate method was adopted for microbial enumeration of TAMC and TYMC. For the plating of sample, 1 mL of diluted sample (1:10 dilution and further dilution if necessary) was aseptically poured into the sterilized petri plate of 90 mm diameter in duplicate. Approximately 20 mL of sterilized SCDA and SDA media, cooled at 45°C were added over the poured sample for TAMC and TYMC respectively and were mixed properly by gently swirling the petri plate in both clockwise and anticlockwise direction and were left to get solidify. The solidified media plates were incubated in BOD incubator at 30-35°C for 3-5 days in case of TAMC and at 20-25°C for 5-7 days in case of TYMC in an inverted position. After completion of the incubation period, petri plates with colony forming unit (cfu) less than 250 for TAMC and less than 50 for TYMC were preferred. The average cfu per g or mL of sample was calculated by selecting the dilution in the respective plates.
Specified Pathogens
Test for the presence of E. coli in the antacids aqueous and non-aqueous preparations were conducted as per the method described in the Indian Pharmacopoeia. For the detection of E. coli, samples were prepared as described above. 10 mL (corresponding to 1 gm or 1 mL) of pre-treated sample was used to inoculate 100 mL of SCDM and incubated for 24 hours at 30-35°C. After 24 hours, the broth was mixed by shaking, transferred 1 mL of the SCDM to 100 mL of MacConkey Broth and further incubated for 24-48 hours at 42- 44°C. After successful incubation, sub cultured on to the MacConkey agar plate from the MacConkey broth. The inoculated plates were incubated for 18-72 hours in an inverted position at 30-35°C. On the MacConkey agar plates, the development of pink, non-mucoid colonies suggest the potential presence of E. coli.
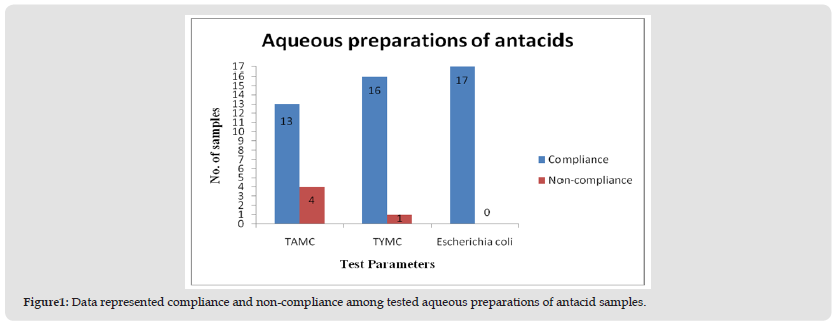
A total of twenty NSP drug samples of antacids (oral suspensions and tablets) were tested. The products physical inspection revealed no signs of unsatisfactory appearance. The samples had a proper manufacturer’s name, manufacturing, and expiry date. There is no foul odor or appearance, neither leakage in the bottle nor any kind of temperedness in the strips. In this study, the main sub-category among the tested samples was the aqueous preparations of antacids, which are orally administered, and they represented 85% (n = 17), while non-aqueous preparations of antacids for oral use constituted 15% (n = 3). The comprehensive analysis of the collected results and their prevalence are presented in (Tables 2 & 3). Among aqueous preparation of antacids, out of 17 samples, four samples were non-compliance as the observed count was beyond the limit. The limit of aerobic microorganisms was exceeded in four samples, while in one sample the limit of molds was exceeded (Figure 1). The noted bacterial count was 2.5 x 107 cfu/mL, 6.0 x 105 cfu/mL, 4.1 x 103 cfu/ mL, and 1.8 x 102 cfu/mL in sample 8, sample 1, sample 6 and sample 13, respectively, while in the case of total yeasts and mold counts, the estimated count was 4.5 x 103 cfu/mL in sample 6. The presence of specified pathogens was absent in all the tested samples. Similarly, for non-aqueous preparations of antacids, none of the samples were under non-compliance.
Table 2: Results of TAMC, TYMC and specified pathogen in aqueous non-sterile preparations of antacids.
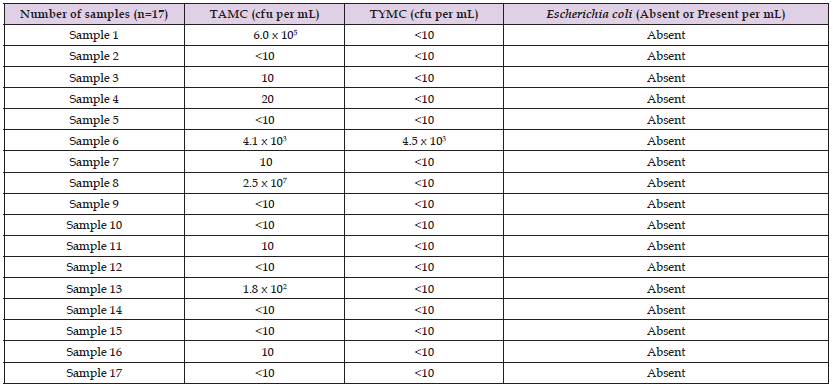
Table 3: Results of TAMC, TYMC and specified pathogen in non-aqueous non-sterile preparations of antacids.
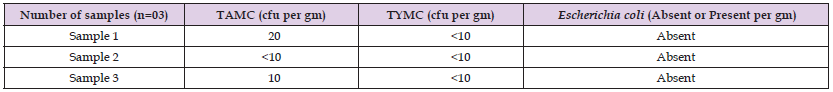
Figure 1 Data represented compliance and non-compliance among tested aqueous preparations of antacid samples.
With the development of pharmaceuticals industries and rising health awareness, pharmaceutical sector play a major role in today’s world [14]. For pharmaceutical preparations to be safe and effective, microbiological quality of compounded medicines is essential. The preservatives ineffectiveness, water source, equipment employed, and post-production storage or transport can all lead to pharmaceutical contamination [9]. High microbial loads have been linked to serious illnesses, especially in immunocompromised people. Moreover, microbial metabolites can alter pH levels and cause drugs to deteriorate, which can make them less potent or even fatal due to the formation of degradation products [15]. As a result, successive editions of the Pharmacopoeia enforce microbial contamination limits in non-sterile preparations to avoid drug-induced infections. Various instances of infections have been cited in scientific literature caused by the usage of tainted medicines. The first ever evidence of a drug-induced infection was observed in 1907, when the vaccine for bubonic plague turned out to be contaminated with tetanus bacilli. Salmonella infection was another case that was recorded, brought on by the presence of Salmonella in tyroidine pills and in pancreatine powder; Pseudomonas cepacia present in iodated povidone, and other cases included sighted illnesses brought on by P. aeruginosa in hydrocortisone ointment, and other items were also recognized [12]. Our findings suggested that the proportion of microbial contamination load in samples collected from market was more in aqueous preparations rather than non-aqueous preparations of antacids. The isolated microbial contaminations were of human flora types, i.e., gram positive or air borne fungi. As antacid is a water-based preparation, microbial contamination of pharmaceuticals has long been a source of concern in aqueous preparations for pharmaceutical manufacturers around the world. It might destroy active components and excipients and cause the formula to putrefy, which will compromise the drug’s potency, stability, and effectiveness. Moreover, a significant health risk emerges for consumers due to the abundance of microorganisms, especially for those who are already unwell or are in vulnerable condition [16]. To reduce microbiological contamination in non-sterile pharmaceutical goods, a variety of activities are required, including GMP, equipment mechanization, monitoring, and post-marketing surveillance [14]. Pharmaceutical quality control is vital not just for standard compliance to maintain the quality of the product but also for reducing risk to the end user [12].
This study has shown that there are some substandard antacid drugs still present in the Indian market that are not of standard quality (NSQ) and can adversely affect the treatment quality and cause problems for patients. To identify any problems linked to product instability, degradation, contamination, or toxin development, several measures, including equipment automation, monitoring programs, and a post-marketing surveillance approach, are necessary. It also recommends strategies and implementation plans to address the issues. Our research findings demonstrate that microbiological quality control is a significant method for improving drug safety, as the potential threat arising from microbiological contamination in pharmaceutical products may alter the active ingredient and even lead to spoilage of the product, as well as cause adverse effects by producing toxins or causing infection. It is also concluded that GMP, GLP, and Pharmacopoeia-compliance microbiological control is essential at all levels of the manufacturing of pharmaceuticals, especially at the stage of the finished goods prior to discharge, and that it must be subjected to prevent contamination during post-production storage in the market. This research article is technical dissertation work to check the microbial contamination in antacids for scientific publication purposes and is not to be used for any legal purposes.
This study is supported by Indian Pharmacopoeia Commission, Ghaziabad, under the Ministry of Health and Family Welfare (MoHFW), GoI, for providing the laboratory facilities.
The authors declared that they have no conflicts of interest.


