Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Jia-Min Xian1,3†, Hong-Tao Hu1,3†, Shu Yang2*, Yu-Na Wang1, Zhuo-Yi Wang1,3, Ruo-Han Zhang1,3, Cheng-Ting Zi1,2*, Pei-Yuan Sun1,2* and Jing Wang1,2*
Received: May 09, 2023; Published: May 16, 2023
*Corresponding author: Cheng-Ting Zi, Key Laboratory of Pu-er Tea Science, Ministry of Education, Yunnan Agricultural University, Kunming, 650201, China and College of Science, Yunnan Agricultural University, Kunming, 650201, China
Pei-Yuan Sun, Key Laboratory of Pu-er Tea Science, Ministry of Education, Yunnan Agricultural University, Kunming, 650201, China and College of Science, Yunnan Agricultural University, Kunming, 650201, China
Jing Wang, Key Laboratory of Pu-er Tea Science, Ministry of Education, Yunnan Agricultural University, Kunming, 650201, China and College of Food Science and Technology, Yunnan Agricultural University, Kunming, 650201, China
DOI: 10.26717/BJSTR.2023.50.007953
Epidermal growth factor receptor (EGFR)-activating mutations are critical factors in EGFR-driven nonsmall cell lung cancer (NSCLC) development, and molecular targeted therapies have focused on inhibiting these mutations. EGFR tyrosine kinase inhibitors (TKIs) have remarkable inhibitory effect for NSCLC with EGFR mutations. However, acquired resistance limited the clinical application of EGFR-TKIs, which highlights the need for discovery of novel inhibitors. In this study, we created Ba/F3 cells harboring EGFR L858R/T790M/G796R mutations, and then found magnolol effectively inhibited EGFR L858R/T790M/ C797S activation, which may be a potential strategy against osimertinib-resistant EGFR mutations.
Keywords: NSCLC; EGFR L858R/T790M/G796R; Magnolol; Osimertinib Resistance
Abbreviations: NSCLC: Non-Small Cell Lung Cancer; EGFR: Epidermal Growth Factor Receptor; EGFR-TKIs: Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors
Non-small cell lung cancer (NSCLC) accounting for 85% of lung cancer patients is one of the cancers with the highest mortality and incidence in the world [1]. Currently, medical approaches targeting oncogenic drivers have reformed the treatment of NSCLC [2,3]. Epidermal growth factor receptor (EGFR) has received much attention, its activation or overexpression has been found in 38% of NSCLC patients [4]. NSCLC patients with EGFR-activating mutations that mostly occur in EGFR tyrosine kinase domain respond well to EGFR-TKIs [5]. The development of EGFR-TKIs brings great clinical benefits to the NSCLC patients with EGFR-sensitive mutations. First- and second- generation EGFR-TKIs, such as gefitinib [6] and afatinib [7], were approved for the treatment of NSCLC patients with EGFR-sensitive mutations, such as deletion in exon 19 and L858R point mutation in exon 21[8]. Among NSCLC patients with EGFR-sensitive mutations, the mutation rate of these two mutations is 51% and 42% respectively [8]. However, despite its substantial benefit and efficacy, NSCLC patients inevitably develop acquired resistance within 9-13 months of treatment [6]. The most common acquired resistance has been disclosed to be a gatekeeper mutation, EGFR T790M mutation in exon 20, which hinders the binding of the EGFR-TKI to the ATP-binding site of EGFR and enhances the ATP affinity of mutated EGFR [9].
EGFR T790M mutation, involving substitution of threonine at position 790 with methionine, has been detected in 50%-60% of resistant case [10]. To address this complex situation, covalently binding irreversible third-generation EGFR-TKIs selectively targeting EGFR T790M mutation while sparing wild type EGFR have been developed and evaluated. Osimertinib (AZD9291), the first third-generation EGFR-TKIs approved by the US Food and Drug administrator (FDA), exhibits remarkable efficacy with controllable toxicities [11]. Taken a progression-free survival (PFS) of 17.8 months [10] and overall response rate (ORR) of 62%-70% [11] into consideration, osimertinib displays better therapeutic effect than first- and second- generation EGFR-TKIs and chemotherapy for advanced NSCLC patients. However, resistance to osimertinib, a serious obstacle, emerged within 10 months [12]. EGFR C797S mutation in exon 20, in which cysteine at position 797 is substituted by serine, has been uncovered in 30% of osimertinib-resistant cases [13]. And C797 is the binding site of osimertinib. Except for EGFR C797S mutation, tertiary EGFR G796R mutation has been confirmed to confer resistance to osimertinib because of the steric hindrance created by the mutual presence of the side chains of EGFR G796R mutation and the osimertinib core [14]. The development of next-generation EGFR-TKIs to overcome osimertinib resistance has become the focus of multiple studies.
Among these, EAI045 [15], an EGFR allosteric inhibitor, has been discovered to be potent against EGFR C797S mutation. A combination of EAI045 and anti-EGFR antibody cetuximab has been found to behave stronger inhibitory effect on EGFR C797S mutation than that of EAI045 alone [16]. Despite that, either EAI045 or EAI045 combined with cetuximab has failed to inhibit EGFR G796R mutation [14]. Moreover, studies on inactivation of EGFR G796R mutation has been less reported. Magnolol, a natural phenolic lignan compound isolated from the root and stem bark of Magnolia officinalis with multiple biological activities, such as anti-cancer, antioxidant, anti-angiogenesis, anti-inflammatory and so on, which has been discovered to be used as Chinese folklore medicine and reported to possess the capability of preventing or inhibiting the growth of various cancer cells [17,18]. In this study, a massive chemical library was screened, and we identified magnolol to be a potential inhibitor of EGFR G796R mutation.
Reagents and Compounds
RPMI-1640 medium, penicillin-streptomycin (P/S) solution and puromycin were obtained from Solarbio (China). Fetal bovine serum (FBS) was purchased from Gibco (USA). All monoclonal antibodies were gained from Cell Signal Technology (USA). 3-(4,5-dimethylthiazol- 2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) was obtained from Promega. The compounds used in the experiment were purchased from Bio Bio Pha (China) and Target Mol (USA).
Cell Culture
WEHI cells were cultured in RPMI-1640 medium supplemented with 10% FBS and 1% P/S. WHEI cell medium were collected and filtered as the source of Interleukin-3 (IL-3). Ba/F3 cells were cultured in RPMI-1640 medium with 10% FBS, 1% P/S and 5% WEHI-conditioned medium. All cells were grown in 5% CO2 and 37℃ humidified atmospheres.
Ba/F3-EGFR(L858R/T790M/G796R) Stable Cell Line
Electroporation method was used to establish Ba/F3 cells stably expressing EGFR-L858R/T790M/G796R mutation. Ba/F3-EGFR( L858R/T790M/G796R) cells were grown in RPMI-1640 medium supplemented with 10% FBS, 1% P/S, 10 ng/mL and 2 μg/mL puromycin.
Flow Cytometry Analysis
Expression of EGFR molecule in Ba/F3-EGFR(L858R/T790M/ G796R) cells was first estimated by flow cytometry.
Cell Viability Assay
Cells were seeded in 96-well plates and cultured overnight. 24 kinds of kinase inhibitors of 1 μM and various concentrations of magnolol were added and the cells were incubated for 48 h. After that, 20 μL of MTS was added and incubated for 3h. The absorbance was measured at 492 nm on a Multifunctional Microplate Reader (Flex Station 3, Molecular Devices, USA).
Western Blotting (WB)
Cells were collected and lysed in lysis buffer (Solarbio, China). The protein sample was then subjected to SDS-PAGE and electro transferred to PVDF membranes (EMD Millipore Corporation). The membranes blocked in 5% non-fat milk, and then were incubated with the antibodies. Proteins were visualized with a Fluor Chem E System (Protein Simple, USA).
Statistical Analysis
Experimental data were analyzed using Graphpad Prism Software. Differences among multiple groups were analyzed using oneway ANOVA. Experimental data were presented as mean ± standard deviation (SD). All the experiments were performed independently at least three times. Significant probability (P)-values are indicated as ***P< 0.001, **P< 0.01 and *P< 0.05.
EGFR L858R/T790M/G796R Introduced into Ba/F3 Cells
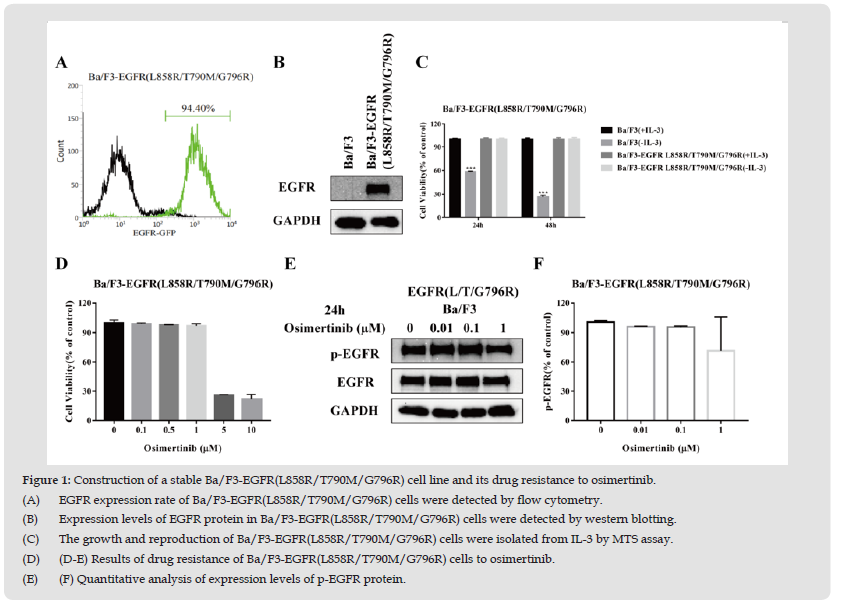
We transfected Ba/F3 cells with EGFR L858R/T790M/G796R using electroporation. The proportions of EGFR-positive Ba/F3 cells were more than 90% following monoclone (Figure 1A) and EGFR proteins were expressed in transfected Ba/F3 cells (Figure 1B). IL-3 is very important for survival of Ba/F3 cells. EGFR is introduced into Ba/F3 cells, which results in the growth of EGFR-driven Ba/F3 cells. Therefore, viability of Ba/F3 cells harboring EGFR is independent of IL-3. As shown in Figure 1C, the viability of Ba/F3 cells was markedly reduced by deprivation of IL-3. The growth of transfected Ba/F3 cells was not significant difference with or without IL-3. Therefore, transfected Ba/F3 cells showed resistance to osimertinib (Figures 1D-1F). These results suggested that L858R/T790M/G796R mutant EGFR was stably expressed in transfected Ba/F3 cells.
Figure 1 Construction of a stable Ba/F3-EGFR(L858R/T790M/G796R) cell line and its drug resistance to osimertinib. (A) EGFR expression rate of Ba/F3-EGFR(L858R/T790M/G796R) cells were detected by flow cytometry. (B) Expression levels of EGFR protein in Ba/F3-EGFR(L858R/T790M/G796R) cells were detected by western blotting. (C) The growth and reproduction of Ba/F3-EGFR(L858R/T790M/G796R) cells were isolated from IL-3 by MTS assay. (D) (D-E) Results of drug resistance of Ba/F3-EGFR(L858R/T790M/G796R) cells to osimertinib. (E) (F) Quantitative analysis of expression levels of p-EGFR protein.
The Effect of Kinase Inhibitors on EGFR-Positive Cell Lines In Vitro
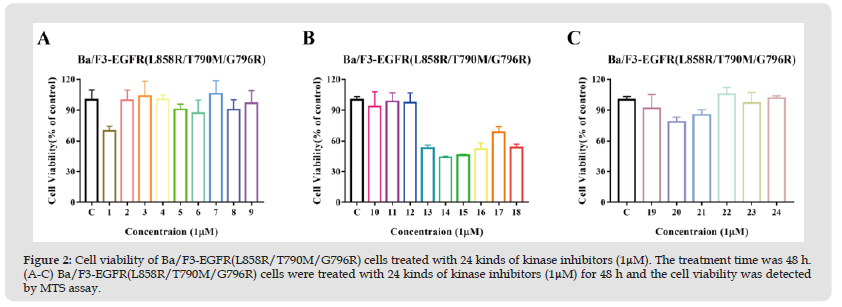
EGFR mutation mostly occurs in kinase domain. Therefore, we examined whether 24 kinds of kinase inhibitors repress the cell viability of Ba/F3-EGFR(L858R/T790M/G796R) cells via MTS assay. As shown in Figure 2, 24 kinds of kinase inhibitors were unable to restrain the viability of Ba/F3-EGFR(L858R/T790M/G796R) cells compared with the control group (Table 1).
Figure 2 Cell viability of Ba/F3-EGFR(L858R/T790M/G796R) cells treated with 24 kinds of kinase inhibitors (1μM). The treatment time was 48 h. (A-C) Ba/F3-EGFR(L858R/T790M/G796R) cells were treated with 24 kinds of kinase inhibitors (1μM) for 48 h and the cell viability was detected by MTS assay.
The Effect of Kinase Inhibitors on EGFR Phosphorylation in EGFR-Positive Cell Line
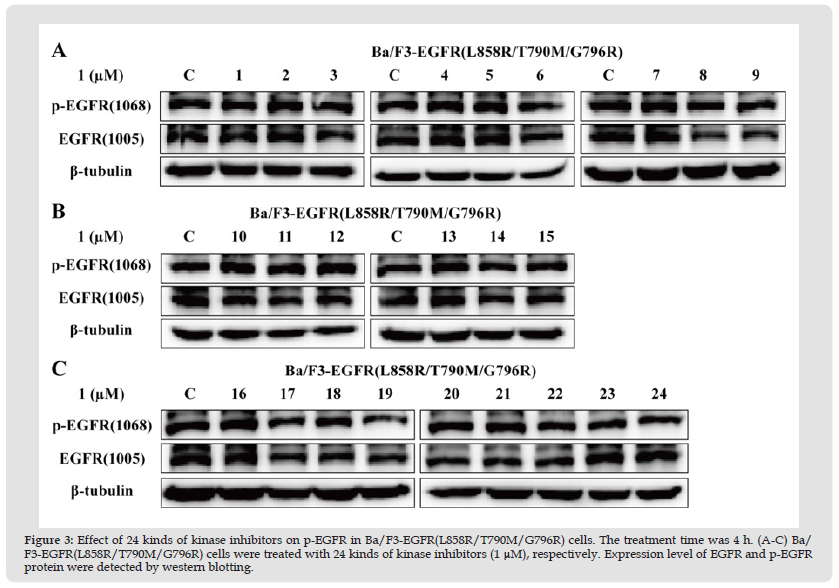
Based on the mentioned results, we continued to verify the effect of 24 kinds of kinase inhibitors on p-EGFR protein expression in Ba/ F3-EGFR(L858R/T790M/G796R) cells by western blotting method. The experiment results were shown in Figure 3, kinase inhibitors were incapable of downregulating the expression level of p-EGFR protein.
Figure 3 Effect of 24 kinds of kinase inhibitors on p-EGFR in Ba/F3-EGFR(L858R/T790M/G796R) cells. The treatment time was 4 h. (A-C) Ba/ F3-EGFR(L858R/T790M/G796R) cells were treated with 24 kinds of kinase inhibitors (1 μM), respectively. Expression level of EGFR and p-EGFR protein were detected by western blotting.
Magnolol Inhibited the Growth of Ba/F3-EGFR(L858R/ T790M/G796R) Cells In Vitro
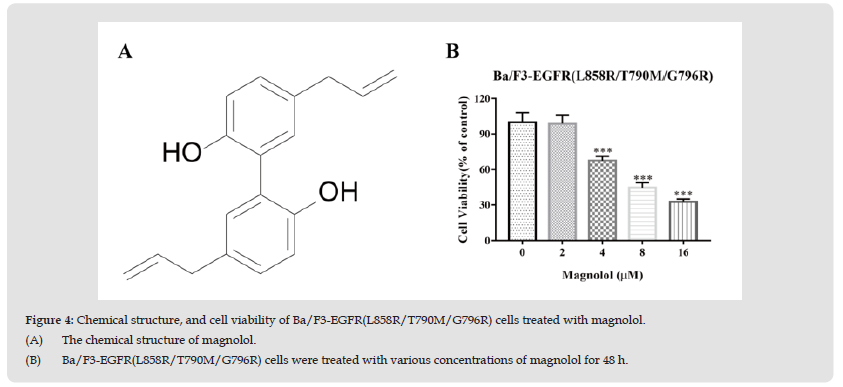
The chemical structure of magnolol is shown in Fig 4A. We then treated Ba/F3 cells expressing triple-mutant EGFR with magnolol. As shown in Figure 4B, magnolol effectively inhibited viability of triple- mutant EGFR cells in dose-dependent manner.
Figure 4 Chemical structure, and cell viability of Ba/F3-EGFR(L858R/T790M/G796R) cells treated with magnolol. (A) The chemical structure of magnolol. (B) Ba/F3-EGFR(L858R/T790M/G796R) cells were treated with various concentrations of magnolol for 48 h.
NSCLC has become one of the most lethal diseases with high mortality and morbidity, which has been threatening human life and health [19]. EGFR is a typical drug target for NSCLC and its mutations have been uncovered in 10-30% of NSCLC patients [5], which have conferred sensitivity to EGFR-TKIs [20]. Despite the wide clinical use of EGFR-TKIs, drug resistance inevitably developed [21]. The appearance of tertiary EGFR mutations has limited the application of third-generation EGFR-TKIs, mainly osimertinib [22,23]. EGFR- L858R/T790M/G796R [14], a rare triple EGFR mutation, has been verified as one of the significant reasons mediating osimertinib resistance. It is worth noting that neither EAI045 combined with cetuximab [24] nor EAI045 [25] is up to suppress the triple EGFR G796R mutation. The development of new generation of EGFR-TKIs and novel combination strategies against tertiary EGFR G796R mutation have been played attention to and warranted. Considering the adverse side effects of chemotherapeutics, natural products with low side effects, easy accessibility, high efficiency and cost effectiveness have been deeply concerned [26]. In this study, we established Ba/F3 cells stably expressing EGFR-L858R/T790M/G796R mutation and got to know that magnolol [27], a natural phenolic lignan compound, was qualified to hinder the cell growth of Ba/F3-EGFR(L858R/T790M/G796R) cells, which has been preliminarily presumed as a powerful candidate for the next generation of EGFR-TKIs.
The Yunnan Provincial Key Programs of Yunnan Eco-friendly Food International Cooperation Research Center Project (2019ZG00904, 2019ZG00909); the Yunnan Fundamental Research Project (202201AU070177); the Yunnan Provincial Department of Education Research Project (2022J0298).
The authors declare that there are no conflicts of interests.


