Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Song Jiang*1, Yue Liu2 and Taoqing Wang3
Received: May 04, 2023; Published: May 16, 2023
*Corresponding author: Song Jiang, Huzhou Institute of Biological Products Co., Ltd., China
DOI: 10.26717/BJSTR.2023.50.007951
A novel biocompatible fast-gelling hydrogel has been explored for neurodegenerative diseases. The hydrogel is formed using thiol-maleimide reaction. The precursors maleimide modified 4-arm polyethylene glycol (PEG) and thiolated hyaluronic acid (HA) were created and characterized by NMR. The hydrogel was investigated by the measurement of gelation time, swelling ratio, viscoelasticity and degradation. Based on the data of gelation time, we found that the hydrogel with two percent maleimide modified 4-arm PEG and thiolated HA was most appropriate for tuning. This percentage of hydrogel was selected for other tests. The tests of swelling ratio and degradation indicated hydrogel was flexible and degradable. The viscoelasticity test revealed that the hydrogel is an elastic solid. From these measurements, this novel biocompatible fastgelling hydrogel could be used for the treatment of neurodegenerative diseases.
Keywords: Hydrogel; PEG; HA; Gelation
Neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and multiple sclerosis, are worldwide issues that could lead to permanent patients’ permanent disabilities or mortalities and huge medical costs [1]. For example, It is estimated that the number of patients with Alzheimer’s disease will reach 14 million by 2050 in America alone [2]. The cost of Alzheimer disease was nearly 200 billion US dollars in 2013 and is expected to be 1.2 trillion in 2050 [2]. Although aging is considered the main cause for neurodegenerative diseases, [3] we still don’t know the detail process of neurodegeneration [4]. Due to the fact that neuron tissue has very limited, if not no, regenerative capacity, most of treatment for neurodegenerative diseases are either focus on delaying the neurodegeneration process or temperately restore the function of the degenerated neuron system by electric shock. The ideology behind these proposed treatments is improving the life quality of the patients rather than a cure [5].
Nowadays, rapid progress in biology and materials development, neural stem cell therapy is a promising approach to treat neurodegenerative diseases. Unlike organ transplantation which with very limited supply and often rises ethnical issues, stem cells are versatile and available from abundant resources with no ethnical issues [6,7]. The stem cell therapy is promising yet still challenging because of some technical barriers, such as the elimination of teratoma formation, unexpected immune responses, transmission of adventitious agents and controlling the stem cell fate including the survival potential, differentiation status, proliferation capacity and migration ability [8]. Based on the result from previous research, the stem cell fate relies on precise spatial and temporal control of biophysical and biochemical micro-environmental cues in the extracellular matrix (ECM) [9]. However, patients suffering from neurodegenerative diseases have no ECM to compensate the neuronal loss. Lack of ECM, which provides crucial physical, chemical and biological micro-environment to regulate the behavior of NSCs, is the main cause of the limitation in the NSCs therapy [10]. Using biomaterials as scaffolds, especially in hydrogel formation, can provide permissive microenvironments to enhance NSCs survival and control NSCs fate, both in vitro and in vivo, by holding and precisely delivering stem cell regulatory signals in a physiological relevant fashion [9,11].
To prepare hydrogels as ECM mimics, various strategies, such as radical polymerization, click chemistry, Schiff base reaction and physical crosslinking, have been developed and investigated and including [12]. Radical polymerization affords well-characterized reaction kinetics and facile in situ polymerization but the free radicals and heat generated during the reaction may impact cells [13-15]. Physical crosslinking strategy has advantages such as simple network formation, non-toxicity, shear-thinninpolymerization, cellent shear recovery but suffers from insufficient mechanical strength and uncontrolled degradation rates [16,17]. Also, So far, there are various kinds of hydrogels based on different kind of biomaterials, such as polysaccharide, polyethylene glycol, zwitterionic, etc. [18-23]. Click reactions are perspective strategies, those can compensate the drawbacks of hydrogels mentioned above [24-27]. Among all click reactions, the maleimide-thiol reaction attracted my attention due to its fast reaction kinetics, high regio- and chemo-selectivity, mild reaction condition and facile tuning of structural and mechanical properties [28-31]. Here, in this article, I explored the applicability of thiol-maleimide reaction to synthesize cross-linked P E G hydrogels. The synthesis of the P E G hydrogels is described herein and characterized in terms of their gelation speed, swelling ratio, viscoelasticity and degradation.
Materials
4-arm PEG, HA, ethanol, dichloromethane, N,N’-dicyclohexylcarbodiimide, cystamine dihydrochloride, 1-(3-Dimethylaminopropyl)- 3-ethylcarbodiimide hydrochloride, hydroxybenzotriazole, tris(2-carboxyethyl)phosphine, hydrochloric acid (HCl) and carboxyl end group maleimide (GMI) were purchased from Sigma-Aldrich.
Synthesis
Synthesis of 4-Arm PEG with Maleimide Groups (PEG-4-MAL): Figure 1 illustrates the synthetic route for creating PEG-4-MAL. In this method, 0.1 mmol 4-arm PEG and 0.48 mmol GMI were dissolved in dichloromethane. The resulting solution was cooled to 0 °C, after which a solution of 0.52 mmol N, N′- Dicyclohexylcarbodiimide in dichloromethane was added, and the mixture was stirred at room temperature for 24 hours. The resulting mixture was filtered, and the filtrate was then precipitated using diethyl ether. The resulting precipitate was dried under vacuum until it reached a constant weight.
Synthesis of Hyaluronic Acid with Thiol Groups (HA-SH): Figure 2 outlines the synthetic route of HA-SH. Typically, 5 mg HA was dissolved in 1 mL purified water and then conjugated with 3 molar equivalents of cystamine dihydrochloride at pH 4.8 overnight, followed by with 1-(3-Dimethylaminopropyl)-3- ethylcarbodiimide hydrochloride and hydroxybenzotriazole for 2 hours. The resulting product was then purified using dialysis tubing against water for 10 days. After purification, the solution was treated with tris(2-carboxyethyl) phosphine to cleave the disulfide linkage of the cystamine component. The solution was then stirred for 2 hours, and the pH was subsequently adjusted to 3.5 with HCl. In the end, HA-SH was precipitated in ethanol, redissolved in purified water, lyophilized for later use.
Preparation of Hydrogel
To form the gel, PEG-4-MAl and HA-SH hydrogel precursors were first dissolved in pH 7.4 PBS, and then the PEG-4-MAl and HA-SH solutions were mixed together. The thiol groups reacted with maleimide groups, resulting in the formation of a three- dimensional structure and the creation of the gel.
Characterization
Characterization of PEG-4-Mal and HA-SH: The molecular structures of PEG-4-Mal and HA-SH were confirmed by Proton and Nuclear Magnetic Resonance. 1H NMR spectra were recorded with an Avance Bruker equipped with BBO z- gradient probe. Experimental
Conditions were as follows: each sample scanned 256 times. Measurement of Gelation Time: To determine the gelation time, the vial tilting method was utilized. Firstly, PEG-4- MAL and HA-SH were dissolved in a pH 7.4 PBS solution, respectively. The hydrogels were then formed by mixing both solutions and quickly vibrating them at room temperature. The gel state was considered achieved if there was no flow observed within 1 minute upon inverting the vial.
Swelling Ratio: A gravimetric method was employed to assess the swelling behavior of the freeze-dried hydrogels. The samples were incubated in pH 7.4 PBS at 37°C until their weight was stabilized. Afterward, the swollen hydrogels were dried with filter paper to remove any excess water and weighed until a constant weight was reached. Using the following equation, the swelling ratio was calculated:
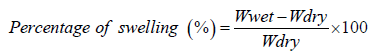
In the context of hydrogel evaluation, the terms Wwet and Wdry denote the weight of the hydrogel when it is wet and when it is dry, respectively.
Degradation: The degradation of the freeze-dried hydrogel samples was investigated by immersing them in pH 7.4 PBS at 37°C for specific time intervals. At each designated time interval, the samples were taken out of the PBS, dried, and weighed. The hydrogel’s degradation was subsequently calculated using the following equation:
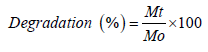
In the provided context, Mt and Mo represent the weight of freezedried hydrogels at the selected time interval and before immersion in PBS, respectively.
Rheological Analysis: The dynamic rheological properties of the hydrogels were assessed using a Discovery Hybrid Rheometer, utilizing parallel plate geometry with an 8 mm diameter. Frequency sweep measurements were conducted at 37°C, ranging from 0.1 to 100 rad/s, with a fixed strain within the linear viscoelastic region.
NMR Analysis
PEG-4-MAL: Confirmation of the PEG-4-MAL structure was obtained through the 1H NMR spectrum, as depicted in Figure 3. The peak observed at 6.9 ppm was assigned to protons in the maleimide group. Additionally, the peaks detected at 3.8–3.4 ppm were attributed to protons in the 4-arm PEG backbone. The degree of functionality of the PEG-4-MAl can be estimated to be around 90% based on the comparison of the integrals of the resonance signals at 6.9 ppm and 3.8–3.4 ppm. The confirmation of HA-SH structure was carried out by 1H NMR, with HA modification being analyzed using the acetamido moiety resonance of HA at δ = 1.95-1.85 ppm as an internal standard. The peaks observed at δ = 2.7-2.5 ppm correspond to cysteamine in HA-SH. The degree of functionality of HA-SH was estimated to be around 10% by comparing the integrals of the resonance signals at 2.7-2.5 ppm and 1.95-1.85 ppm. Table 1 summarizes the gelation time of hydrogels. The weight percent of hydrogel precursors used ranges from one percent to four percent. The gelation time varies from ∞ to one second. Only the two percent PEG-4_MAL and two percent HA-SH formula is feasible because longer or shorter times could lead to cell encapsulation failure. For instance, longer times mean lower weight percent of hydrogel precursors used, which could form an amorphous gel. Conversely, shorter times could result in incomplete “clicking” of the maleimide and thiol groups, which would affect cell growth. Therefore, this formula was used for the remaining experiments and analysis (Figure 4).
Degradation Analysis
Figure 5 shows the weight loss trend of the hydrogels over time. The weight loss speed of the hydrogels was observed to increase with time. Specifically, the weight loss percentages were 26%, 45%, and 85% on the 1st, 3rd, and 5th day respectively. By the 7th day, the hydrogels had degraded more than 90%, and by the 9th day, they had degraded completely.
Swelling Analysis
Figure 6 presents the results of the swelling ratio with different gram samples. The freeze-dried samples ranged from 1 gram to 5 grams. Despite the varying weights, all samples exhibited similar swelling ratios, exceeding 94%. The hydrophilicity of the samples influenced the amount of water absorbed upon submersion in PBS, resulting in higher swelling ratios. Notably, the two percent PEG-4MAL and two percent HA-SH hydrogels absorbed an equivalent weight of water as the freeze-dried hydrogels, indicating good flexibility and ECM mimic of the hydrogels produced using this formulation.
Rheological Analysis
Figure 7 displays the plot of G′ and G′′ as a function of angular frequency for the hydrogels, which exhibited characteristic viscoelastic behaviors. The moduli G′ were consistently higher than G′′, demonstrating that hydrogels formed by maleimide-thiol coupling chemistry have an elastic solid nature.
This article presents the development and characterization of a new biocompatible fast- gelling hydrogel created from functionalized HA-SH and functionalized PEG-4-MAL via the maleimide-thiol reaction. NMR analysis confirmed the successful synthesis of the HA-SH and PEG-4-MAL precursors. The hydrogels were evaluated based on the gelation time, swelling ratio, viscoelasticity and degradation. The optimal hydrogel formulation was determined to be two percent HASH and two percent PEG-4-MAL based on the gelation time analysis, which was used for subsequent testing and analysis. The hydrogels exhibited good flexibility and ECM mimicry as demonstrated by the swelling ratio. The degradation tests showed complete degradation of the hydrogels after 9 days. The viscoelasticity test demonstrated that the hydrogels formed by maleimide- thiol coupling are elastic solids. These findings suggest that this novel biocompatible fast-gelling hydrogel holds potential for use in the treatment of neurodegenerative diseases.


