Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Akbar mobaraki1, Saghar Sakhaee2, Sahar Sakhaee3, Mohammad Hossein Sakhaee4, Ahmad Takallou5 and Nader Sakhaee6*
Received: April 28, 2023; Published: May 09, 2023
*Corresponding author: Nader Sakhaee, School of Chemical Sciences, University of Illinois Urbana Champaign, IL, USA
DOI: 10.26717/BJSTR.2023.50.007927
Introduction of ring flipping concept for chair conformers in cyclohexane, and the isolation of its twist conformer on a cold Cesium plate, has so far revolutionized our understanding of conformations in medium-sized rings and even small cyclic molecule, like the fluxional cyclopentane. Chemists have since built models and pondered conformational dynamics in cyclic molecules. The wheel-model of Lipnick, et al. as well as the onion models of Hendrickson et al are among the most sensible and sophisticated models, which gained new insight in conformational dynamics of cyclic molecules. Here, DFT-ωb97xd/ 6-311+G* computations confirmed by MP2/aug cc-pVTZ computations were used to fully investigate the energy, vibrations, and full dynamics of 42 conformers. Then spherical conformational landscape SCL model was used to plot the conformational dynamics in cycloheptane for the first time. Classifications were done by a close inspection of the whole cast of conformers in cyclopentane and cyclohexane. The fluxional Twist/ Boat T/B ring coordinates were found to be a common feature in all the cyclic molecules studied. A new pictorial notion is suggested to present twist and boat conformers for all cyclic molecules in a generic sense. The simple, precise and universal conformational types presented here, unify our perception of conformational dynamics in cyclic molecules, and paves the path towards a better analysis of cyclic motifs in natural products and bio-functional conformers.
Keywords: Ring Flipping Concept; Spherical Conformational Landscape Model; Onion Models; Medium Sized Rings; Twist/Boat T/B Ring Coordinates; Wheel-Model; Bio-Functional Conformers
Conformational analysis of Cyclic compounds has evolved into simple models like ring flipping in cyclohexane [1-6] all the way to dynamic models like the wheel model [7] and pseudo rotation for cyclopentane. Pseudorotation was pioneered by Lipnick’s, et al. [8] model, followed by Laane, et al. [9-11] and further elaborated by Leutwyler, et al. [12,13] implicitly using pseudorotation on ring coordinates. Common conformer nomenclature is mostly rooted in cyclohexane models [12,14-17] presented in organic chemistry textbooks including twist, twist-boat, chair, half chair, and envelope. While for medium sized rings C7-C10 there seems to exist no consensus both in naming and dynamic conformational interconversion. Instead, a jumble of conformers are presented with complicated puckering and dihedral analysis has been used trying to address those rings. One very fine account on medium sized rings is presented in a series of papers by Hendrickson, et al. [18-22] to provide a full analysis. Hendrickson believes that “In pseudo-rotation all the bonds change dihedral angles, the deformation is roughly equalized around the ring, and only negligible if any bond-angle changes are necessary, with the result that pseudo-rotation barriers are relatively small.” This awesome description of the process is what we try to build a the very common feature of Cycloheptane in this paper. In 2016 the Spherical conformational landscape model was presented [23] to classify conformers based on their energies to fully address the pseudo rotation in cyclopentane, similar patterns can also be found boat-twist conformational dynamics for cyclohexane.
In this paper a Spherical conformational landscape model is successfully defined for cycloheptane. The purposed model relies on two simple classes of conformers common to all ring compounds based on CS and C2 symmetries. We try to define the PES referred to as the conformational landscape hereafter by two types of simple ring coordinates. These Bending and twisting ring coordinates are strongly backed up by DG band in Raman spectra. And are of prime importance for addressing the carbon hybridization used to measure purity of carbon nanotubes. This paper first introduces for the first time a symbolic notion for defining all twist and boat conformers used in all cyclic compounds in a unified helpful way to classify and characterize these conformers. Then we use the notion to define conformers in cycloheptane in a the easiest most revealing pattern, Spherical conformational landscape model (SCL model), trying to highlight the big picture. In doing so we believe that pseudo rotation exists as a dynamic process in all cyclic compounds from cyclopentane to all medium- sized rings. Pseudo rotation is in essence, a series of molecular vibrations that convey the puckering around the ring’s circumference in an efficient form to reduce Bayer strain.
Computations were all done at the DFT-ωb97xd/ 6-311+G* level of theory [24-28], using gaussian g09 package [29], the reliability of method was further checked for some conformers at both extreme of stability with MP2/aug cc-pVTZ. The choice for DFT-ωb97xd/ 6-311+G* was made due to our previous reports on cyclopentane and cyclohexane with reliable C-H bond length as well as the obtained geometries. MP2/aug cc-pVTZ , as were the case with cyclopentane, offered very slight changes and huge computational cost for the numerous conformers studied. Computations were done for all conformer of cyclooctane as well as frequency computations. Intrinsic Reaction Coordinate (IRC) computations were preformed to gain insight into the conformational dynamics of B(3.5) , T(2-2,1), chair-envelope and T(3,2-1) conformers. The data was used to assemble the resulting ring coordinates into a conformational landscape model for cycloheptane. Among the ring the T(2-2,1) ring coordinate is much faster than the T(3,2-1) ring coordinate. Thus, the higher energy of T(2-2,1) compared to T(3,2-1) may well suggest a temperature dependent fluxional behavior for cycloheptane.
Pseudo rotation was first recognized in cyclopentane molecules with its unusually high gaseous entropy, and it has been tagged with cyclopentane fluxional nature ever since. The fluxional nature of cyclopentane is not a unique attribute as previously claimed, 8, 10 rather it’s a result of the introduced torsion in twist and bent conformers for cyclohexane and cyclopentane respectively. In cyclohexane the Boat/Twist or B/T ring coordinates are not populated that much due to more stable chair conformers that are highly populated in the ground state. However, for cyclopentane, all possible conformers are on the B/T ring coordinates, trapping this beautiful molecule in a constant ultrafast flip flop known as pseudo rotation. Textbook of organic chemistry usually present a cast of conformers for cyclohexane and a couple for cyclopentane presented from different viewpoints designed to serve an educational purpose ( see SI). However, there could be a universal topical view that can bear all the information about a particular conformer and then other viewpoints can be tagged to such general notion in other specific topics. Such general views are introduced here for cyclopentane, cyclohexane for which the SCL models are given in SI. The same logic of nomenclature and presentation is exactly is used to introduce all conformers for cycloheptane. The newly introduced system is then further used to elaborate on the nature of cycloheptane conformers compared to cyclohexane and cyclopentane.
As previously mentioned, there are two major classes of dynamic conformers for any cyclic molecule of interest. Relatively static, usually more stable conformers are found in even numbered rings like chair cyclohexane or crown cyclooctane. Both of which are located at the poles in the SCL model and feed the pseudo rotation in the ring coordinates (see SI). For both the twist and boat conformers the twisted or bent midsection with maximum puckering is taken as a reference line (see SI). Moving in towards this reference line/s we count the number of bonds touching both flanking sides and then the bonds touching the midsection area of the twist form, and combine them as digit codes in parentheses to tag a unique twist or boat form of special character (for boats there is only one number as they all have a plane of symmetry and two equal flanking sides, thus numbers on both sides would always be equal. Apart from simplicity, the usefulness of such a notion is that the emerging pattern of inter ring conversions are dictated by the character of its constituent conformers. One can see that for cyclopentane the famous envelope conformer is a B(2.5)while the bent conformer is a T(2-1,0). Conformers in cyclohexane are defined as B(3) and T(2-1) as well. And finally the conformers for cycloheptane can be defined as B(3.5), T(3,2-1) and T(2-2,1). There are ten T(2-1,0) and ten B(2.5) conformers forming the B/T ring coordinates in Cyclopentane, and twelve (six B(3) and six T(2-1)) such conformers forming B/T ring coordinates in cyclohexane (see SI). Puckering can travel along the ring through the dynamic conversions of this conformers, with the more twisted conformers capable of distributing this puckering faster. The SCL model is used to figure out such puckering distribution in terms of degree per each conversion. Pseudo rotation propagates in cyclohexane by 30o/conv and for cyclopentane 54o/conv. Where in latter, combined with the lack of a stable chair like conformer a fluxional nature is well predicted. The highly twisted T(2-1,0) translates into high energy conformers with fast low barrier dynamic motion.
Table 1. Relativse Energies, vibrational frequencies, and interconversion barriers of cycloheptane conformers.
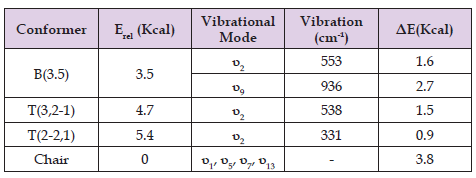
For cycloheptane the inter conversions of the two twists and the only boat forms can easily be found in the vibrational patterns. The B(3.5) conformer can reach either T(3,2-1) or T(2-2,1) with two twisting modes (Figure 1) with higher barrier for T(2-2,1) (Table 1).There is also a breathing mode for the two-twist mode that readily interconverts the two while this same breathing mode for T(2-1) in cyclohexane merely serves to disturb the symmetry as shown. Access to the dynamic pseudo rotational platform is reached from the chair conformer via an activation of 3.5 Kcal making cycloheptane a rather fluxional one. Based on the cast of conformers provided the SCL model for cycloheptane can be constructed, in which three concentric rings stands for the three above mentioned B/T conformers (Figure 2). Each ring contains 14 conformers tagged with a small letter, adding up to 42 conformers which makes the overall 56 counting the 14 chair conformers as well. One can then judge the phase (φ) distribution speed in terms of degree per each conversion. For the slower T(3,2-1) ring starting from a conformer(going clockwise) to e´ and to b back to a makes a full cycle of conversion with 10, 20 and finally 28 conversions in 3 rounds (Figure 2). Thus, the speed of puckering distribution for is 39o/conv, making it faster from cyclohexane but way slower comp ared to pseudo rotation in cyclopentane. However, for the faster T(2-2,1) ring starting from a conformer (going clockwise) to e´ , to b , to f´ and to d´ back to a makes a full cycle of conversion with 6, 12,18, 22 and finally 28 conversions in 5 rounds (Figure 2). The speed of puckering distribution for T(2-2,1) is 64o/conv, making it even faster than cyclopentane. However, the T(2-2,1) is less populated compared to T(3,2-1) conformers. Nevertheless, the model predicts a temperature dependent fluxional nature in cycloheptane with stronger pseudo rotation at higher temperatures. The SCL model is not only a powerful unifying scheme for cyclic molecules but is also can provide insights into conformational dynamics of natural products containing cycloheptene ring and its derivatives, which needs more studies on drug candidates and other molecules of potential interest to biochemists.
In conclusion this work showed that fluxional nature may not be unique to cyclopentane but can rather exist in all cyclic molecules as precisely presented here for cycloheptane. Pseudo rotation thus, may well be a common feature shared in similar conformational landscapes like cyclopentane, cyclohexane as well as the illustrated case here for cycloheptane and other medium sized rings. The SCL model for cycloheptane is to best of our knowledge a first-time representation. We also tried to introduce for the first time a generic notion of defining conformers in cyclic molecules which can tag and address their puckering in the easiest way which we believe eliminates many current redundancies around these conformational forms in current organic chemistry textbooks and literature. The SCL model was further interrogated to comment on the speed of puckering distribution that helps reduce torsional stain in all cyclic molecules. The ability to bold certain dynamical behavior in medium sized rings, with their ubiquitous presence in many natural products can lead to a deeper understanding of their shape and function which will bridge a vital pathway to drug discovery research. Ongoing MD studies on the exact physical origin of fluxional nature would potentially reveal the underpinning dynamics for cyclic molecules and their beautiful chemistry soon.


