Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Jin Peng*
Received: April 25, 2023; Published: May 08, 2023
*Corresponding author: Jin Peng, School of Chemistry and Chemical Engineering, Linyi University, Linyi 276000, China
DOI: 10.26717/BJSTR.2023.50.007922
In this paper, we studied the solubilization of two nonsteroidal anti-inflammatory drugs (Meloxicam and celecoxib) in three Triton X micelles by molecular dynamics simulation. The difference of solubilization between two drug molecules in three micelles was emphasized and the best drug delivery vector was selected.
In the pharmaceutical field, nanoscale DDV has been widely used due to its advantages of greater drug solubility, lower toxicity and more targeted drug delivery [1]. Micelles formed by the aggregation of surfactants have strong solubilization and drug loading ability. They are often used to improve the solubility of drugs with poor water solubility and are commonly used as drug delivery carriers [1-3]. Non-ionic surfactants with less toxicity are attractive candidates for drug delivery carriers due to their biodegradability [2]. In recent years, TX-5, TX-114 and TX-100 nonionic surfactants have been used as effective drug delivery carriers, including solubly-enhancing NSAIDs, antidepressants and anticancer drugs [4-6]. Especially, as the delivery carrier of NSAIDs, it has received wide attention. Many researchers have studied in detail the solution-enhancing and encapsulation effects of non-ionic surfactants of the Triton X family, especially TX-100 and TX-114, on water-insoluble drugs. For example, Chakraborty, et al. [6] studied the interaction between Meloxicam and Triton X-100 micelles by spectroscopic method. The results show that the environment of the drug, namely the micellar properties, plays a decisive role in the selection of a particular proto-form of drug binding. In many pharmaceutical preparations, amphiphilic substances are often used together as active compounds because interactions affect the physicochemical properties of the dosage form, which in turn may alter the stability of the preparation.
In the course of Rub, et al. [7], 1H NMR data showed that ibuprofen interacts with TX-100 micelles through hydrophobic and hydrophilic interactions. In order to improve the bioavailability of NSAIDs, other researchers used TX-100 and TX-114 micelles with high solubility as solvents. For example, Ullah, et al. [8] used spectrophotometry to measure the solubility of flubiprofen in these two micelles and found that both of them could improve the solubility of flubiprofen. In addition, the number of ethylene oxide units in the surfactant plays a key role in the solubilization of meloxicam and celecoxib [9]. In order to further explore the solublization process of NSAIDs in TX micelles at the microscopic level, some relevant researchers used molecular dynamics simulation to conduct detailed observations. For example, Ishkhanyan, et al. [1] reported the effects of ibuprofen and indomethacin on the structural properties of TX-100 micelles. It was found that micelles loaded with ibuprofen had poorer sphericity than those loaded with indomethacin. Subsequently, Ishkhanyan, et al. [2] explored how the solubility of ibuprofen and indomeacin sodium salts drove the changes in the morphology and structure of TX-114 micelles and the solubility process of drugs. The results showed that the two drug molecules were mainly soluble in the hydrophobic core of micelles, but were also largely soluble in the hydrophilic parts of micelles. NSAIDs are mainly used to treat pain and inflammation [2] and have certain anti-inflammatory effects in rheumatoid arthritis [10,11]. In this paper, whole atom molecular dynamics simulation was used to study the solubilation of two water-insoluble NSAIDs, Meloxicam (C14H13N3O4S2) and celecoxib (C17H14F3N3O2S) in the micelles of TX-5, TX-114 and TX-100, respectively, and their effects on the structural properties of the three micelles. Triton X micelle with the best encapsulation effect was selected from six drug-micellar systems.
All systems were studied by whole-atom molecular dynamics simulation, and the simulation software was Gromacs [12]. The force field used was Gromos 54a7, and the molecular structure of Meloxicam and celecoxib was optimized in ATB [13,14]. The water molecular model adopted is single point charge (SPC). Two drug molecules were randomly placed in three micellar systems. The box size of each system is 10×10×10 nm3. The specific components of the six systems are shown in (Table 1). The energy of each system is minimized by using the steepest descent algorithm, so that the initial structure of each system is reasonable. Then, in the process of NVT equilibrium, the temperature of each system reaches 298 K, and then 100 ps NPT equilibrium is carried out. The pressure of each system was stabilized at about 1 bar by using the Parrinella-Rahman pressure controller [15,16]. After reaching the simulation environment closest to the experimental conditions, molecular dynamics simulation of 200 ns was carried out. A time step of 2 fs was used in simulated environment variables, periodic boundary conditions were adopted in all directions, and hydrogen bonds were restricted by LINCS algorithm [17].
Table 1: Specific composition of micelle-drug systems TX-5-MELO, TX-5-CELE, TX-114-MELO, TX-114-CELE, TX-100-MELO, TX-100-CELE.
Micellar Shape, Size and Solvent Accessible Surface Area After Solubilized Meloxicam and Celecoxib
The changes in the size and structure of polymers are key to determining the mechanism of micellar structure [18]. Therefore, we studied the changes in the shape, size and structure of three micelles after solubilizing two drug molecules.
Micellar Shape After Solubilized Meloxicam and Celecoxib: The shape of micelles is often characterized by the value of moment of inertia Imax/Imin, in which Imax and Imin are the maximum and minimum moment of inertia along the x, y or z axes, respectively. The closer the value of Imax/Imin is to 1, the more spherical the micelle tends to be [19]. (Tables 2 & 3) show that meloxicam solubility has no effect on the shape of TX-5 micelles but increases the ellipsoidal degree of TX-114 and TX-100 micelles. The solubility of celecoxib increased the ellipsoidal degree of TX-5, TX-114 and TX-100 micelles, and the increase of the ellipsoidal degree of TX-114 micelles was more obvious. In addition, eccentricity is also usually used to determine the shape of micelles and is defined as e = 1-Imin/Iavg [19], where Imin and Iavg represent the minimum and average moment of inertia of micelles, respectively. The closer e is to 0, the more spherical the micelle is. The eccentricity results show that the changes of the shape of the three micelles by meloxicam and celecoxib are consistent with the results obtained from the Imax/Imin value of the moment of inertia. According to these two indexes, the sphericity of the three TX micelles after meloxicam and celecoxib were increased according to the sequence TX-100 TX-114 TX-5 and TX-114 TX-100 TX-5, respectively.
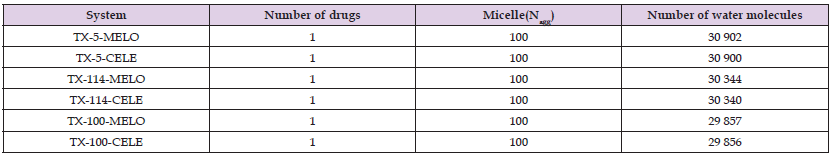
Micelle Size After Solubilizing Meloxicam and Celecoxib: The size of micelles is one of the basic structural characteristics of micelles, and Rg is usually used to characterize the size of micelles [20]. The calculation results in (Tables 2 & 3) show that meloxicam slightly reduces the size of TX-5 micelles but has little effect on the size of TX-114 and TX-100 micelles. Celecoxib reduces the size of all three micelles. Similarly, the average micellar radius Rs is also an important standard for judging micellar size, defined
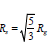
Table 2: Structural characteristics of micelle-drug systems Triton X-5-MELO, Triton X-114-MELO, Triton X-100-MELO.
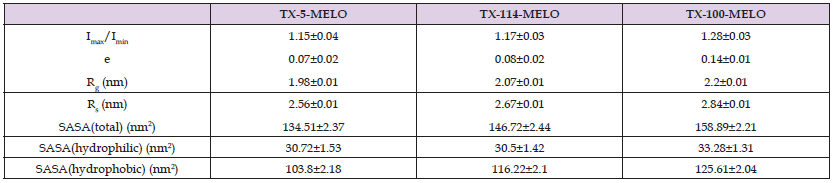
Table 3: Structural characteristics of micelle-drug systems Triton X-5-CELE, Triton X-114-CELE, Triton X-100-CELE.
Surface Area of Solvent Contact: Solvent contactable surface area (SASA) is an important criterion for micellar surface properties, referring to the area where the TX micellar is in contact with the solvent. It is determined using the bicubic lattice method [20] (DCLM), in which all water molecules in the system are removed and A small radius probe, usually 1.4 A, is used to represent one water molecule. The maximum contact area with the surface is determined by rolling [1,21]. In order to observe the effects of two drug molecules on three micellar SASA, we calculated the SASA of each micellar drug complex. It can be observed from (Tables 2 & 3) that the total SASA increased after meloxicam and crecoxib were solubilized by TX-5 micelles, whereas the results of TX-114 and TX-100 micelles were contrary. After meloxicam was dissolved, the total SASA of the three TX micelles, the SASA of the hydrophilic part and the SASA of the hydrophobic part increased according to the sequence TX-5<TX-114<TX-100,TX-114<TX-5<TX-114<TX-100,TX-5<TX-114<TX-100, respectively. After solubed celecoxib, the total SASA of the three TX micelles, the SASA of the hydrophilic part and the SASA of the hydrophobic part increased according to the sequence TX-5<TX-114<TX-100, TX-114<TX-5<TX-114<TX-100, TX-5<TX-114<TX-100, respectively.
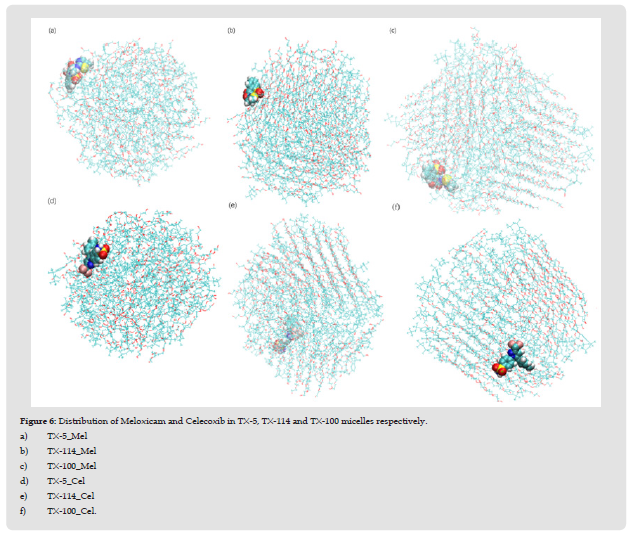
Distribution of Meloxicam and Celecoxib in Three Micelles
In order to observe the distribution of meloxicam and celecoxib in each micelle in detail, this paper characterized the specific distribution of meloxicam and celecoxib in each micelle by calculating the probability distribution of the polar and non-polar atoms in two drug molecules and three TX micelles, as well as the distance between the polar and non-polar groups and the micelle centroid of two drug molecules, as shown in (Figure 1). These are shown in (Figures 2 & 3). The polar and non-polar atomic labels of selected drug molecules and TX micelles are shown in (Figure 4). As can be seen from (Figure 1), polar S atoms and non-polar C atoms in Meloxicam and celecoxib were mostly distributed in the outer regions of each micelle, and TX-100 micelles had the best inclusion effect on the two drug molecules. The distribution of polar head O atoms and tail chain end carbon atoms in the three TX micelles extends from the core of the micelle to the shell region of the micelle, and are also distributed in the surface of the micelle with a high probability, which is similar to the distribution of atoms in the pure micelle before solubilized drug molecules. It is more obvious that the polar head O atoms and tail carbon of TX-5 micelle are mostly located in the outer region of the micelle. (Figures 2 & 5) show that meloxicam is closer to the micellar surface than celecoxib, that is, meloxicam is more infiltrated in the water layer of micellar.
Figure 1 Probability distribution of the distance between the polar atoms and nonpolar atoms in the drug molecule and TX molecule in the drugmicelle system and the micelle centroid (COM).
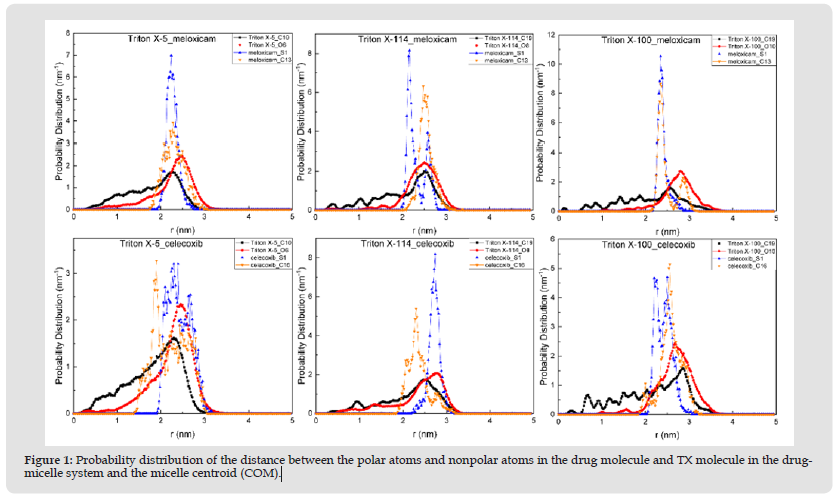
Figure 2 Probability distribution of distance from drug molecule to micelle centroid (COM) in each drug-micelle system.
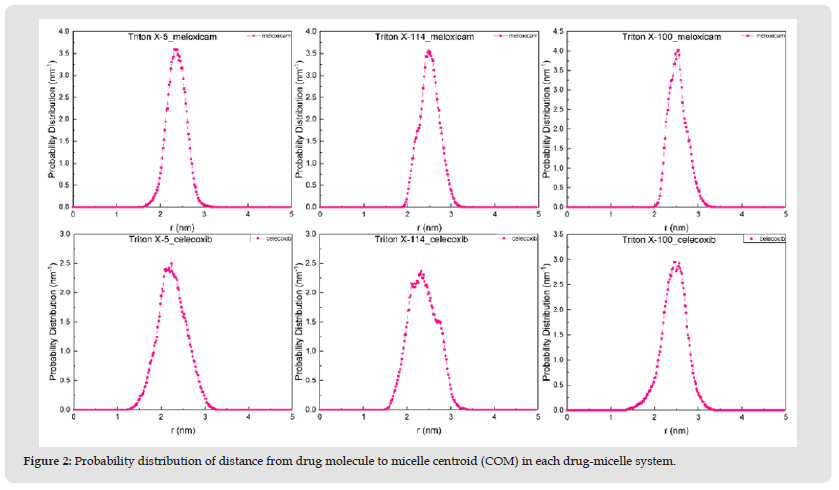
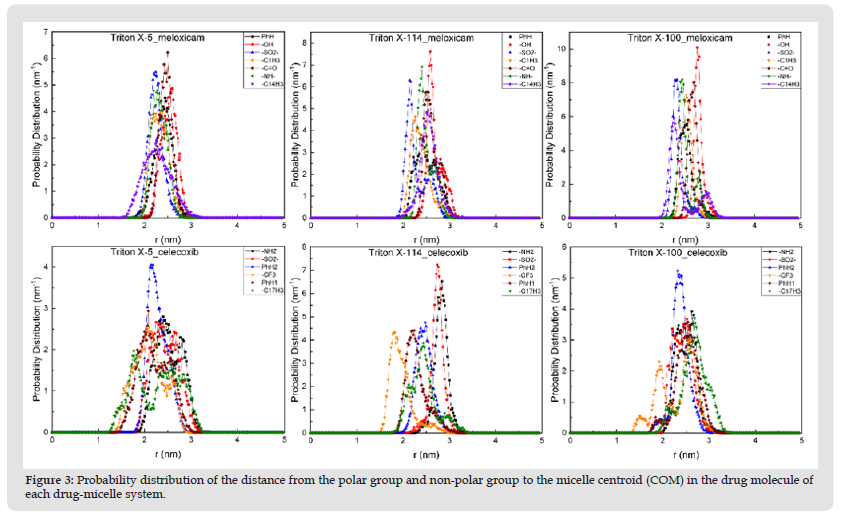
Figure 3 Probability distribution of the distance from the polar group and non-polar group to the micelle centroid (COM) in the drug molecule of each drug-micelle system.
Note: 1, 14 and 17 in - C1H3, - C14H3 and - C17H3 refer to the label of C atom.
Note: Meloxicam (left), Triton X (center) and Celecoxib (right).
Figure 5 Probability distribution of O atoms in H2O in TX-5_Mel, TX-114_Mel, TX-100_Mel and TX-5_Cel, TX-114_Cel, TX-100_Cel micelle-drug systems with respect to micelle COM.
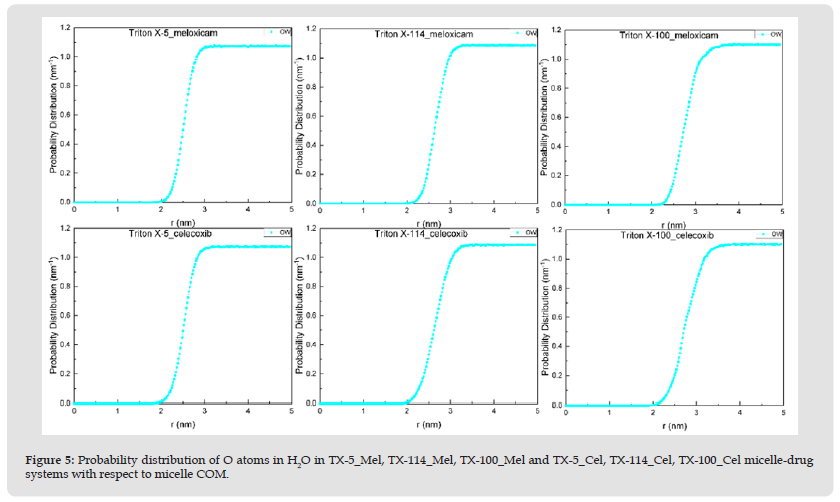
Note: OW stands for O atom in water molecule.
We further explored the distribution of hydrophilic and hydrophobic groups of the two drug molecules in micelles (Figure 3) and found that the -OH in meloxicam was located in the outermost micelle, followed by -C=O, which was found in all three micellar systems. The most medial group in the micelle is -C14h3, -SO2- and -C14h3 in the TX-5, TX-114 and TX-100 systems, respectively. The group in the outermost micellar of celecoxib is -NH2 in both TX-5 and TX-114 micellar systems, followed by -SO2-, and the outermost group in TX-100 micellar systems is -C17H3, followed by -NH2. The most medial group is -C17H3, -CF3, and -CF3 in the three micellar systems. Both polar and non-polar groups of the two drug molecules have their own characteristics in the distribution of the three micellar systems, that is, their distribution positions and the probability distribution of the distance from the micellar centroid are different.
Structure of Each Drug-Micellar System: In the study of Ishkhanyan, et al. [1,2], the distribution of the other two NSAIDs - ibuprofen and indomethacin in TX-114 and TX-100 micelles is also different. They found that about 66% of indomethacin molecules were dissolved in the hydrophobic core of TX-114 micelles, and the rest 34% were located in the outer region of the micelles; About 64% of ibuprofen molecules were dissolved in the hydrophobic core of TX-114 micelles, while the rest were dissolved in the hydrophilic region of the micelles. Ibuprofen molecules are mostly distributed in the core of TX-100 micelles. In order to further clearly characterize the distribution of two drug molecules in each micelle, we selected the configuration diagram of each micellar - drug system, as shown in (Figure 6). As can be seen from the figure, the distribution positions of meloxicam and celecoxib in the three micelles are different. In general, Meloxicam and celecoxib are almost distributed in the surface layer of micelles in the three systems respectively. This corresponds to the probability distribution of the two drug molecules relative to the COM distance in the three TX micelles shown in (Figure 2). Moreover, the shape and size of each micelle are consistent with the results obtained in (Tables 2 & 3). It shows that their microenvironment is also different from each other, which makes great difference in the structural characteristics of the three TX micelles.
Figure 6 Distribution of Meloxicam and Celecoxib in TX-5, TX-114 and TX-100 micelles respectively. a) TX-5_Mel b) TX-114_Mel c) TX-100_Mel d) TX-5_Cel e) TX-114_Cel f) TX-100_Cel.
Interactions Between Drug Molecules and Micelles
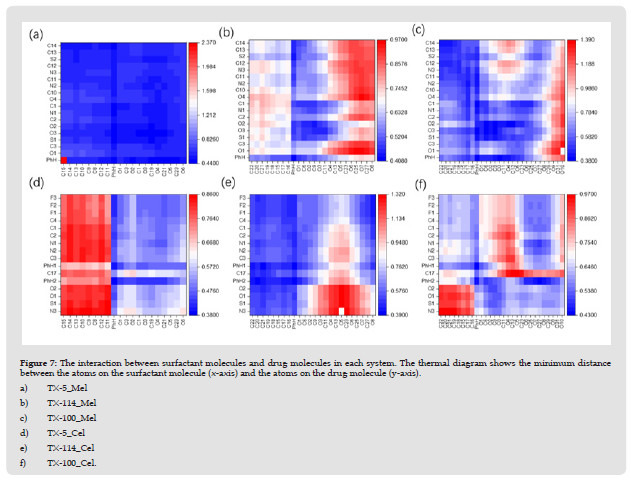
In order to utilize Triton X micelles as a vehicle for drug delivery, it is important to study the details of drug-micellar interactions [22]. In this paper, the interaction of Meloxicam and celecoxib with the three micelles was characterized by measuring the minimum distance between heavy atoms (non-hydrogen atoms) in the drug molecule and the surfactant molecule. The smaller the minimum distance value, the greater the probability of interaction. The benzene ring in the drug molecule and TX molecule is taken as a whole, and one of the C atoms in the PEO chain with similar chemical environment is selected as the research object, as shown in (Figure 7). It is apparent that meloxicam strongly interacts with the TX-5 micelle, corresponding to C14-O6 atoms on the horizontal axis and O1-C14 atoms on the vertical axis in (Figure 7a), indicating that meloxicam is tightly bound to the TX-5 micelle. The interaction between benzene ring and meloxicam was more prominent in TX-114 micelle, and the interaction between EO unit close to the surface of the octyl chain and the PEO chain and meloxicam was weakened in turn. The interaction between octyl phenyl and meloxicam in TX-100 micelle is the most obvious in this system, and the interaction between EO unit on the surface of the micelle is the weakest. An interesting phenomenon was found in the TX-5 Cel system, that is, celecoxib had the weakest interaction with the octyl-part of the TX-5 micelle and the strongest interaction with the benzene ring. In TX-114 Cel system, the octylphenyl chain is the strongest part of the interaction between TX-114 micellar and celecoxib. It can be seen from (Figure 7f) that the octyll in the TX-100 micelle interacts weakest with some of the polar atoms in celecoxib (corresponding to the N3-O2 atom on the vertical axis). In general, two drug molecules dissolved in three micelles have different polar and non-polar interaction regions with different micelles. The results showed that Meloxicam and celecoxib had different binding modes with each micelle.
Figure 7 The interaction between surfactant molecules and drug molecules in each system. The thermal diagram shows the minimum distance between the atoms on the surfactant molecule (x-axis) and the atoms on the drug molecule (y-axis). a) TX-5_Mel b) TX-114_Mel c) TX-100_Mel d) TX-5_Cel e) TX-114_Cel f) TX-100_Cel.
Note: See (Figure 4) for the label of each atom.
Interactions Between Drug Molecules and Water
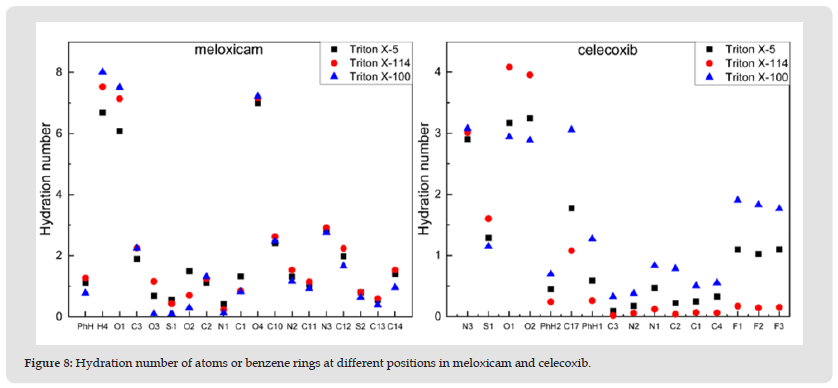
Hydration Number: The interaction between meloxicam and celecoxib in each micelle and environmental water is characterized by calculating the hydration number of atoms or benzene rings at different positions in meloxicam and celecoxib, as shown in (Figure 8). Calculate the hydration number around the selected atom or benzene ring using the position of the first minimum value in each RDF (0.35 nm) [2,23,24]. It can be seen from (Figure 8) that the hydration number of atoms or benzene ring in different positions of the two drug molecules is different in the three micelles, among which, the hydration number of hydroxyl group in Meloxicam reaches the maximum value, while that of N1 atom is the lowest. This phenomenon occurs in all three systems. The hydration number of the polar head group in celecoxib was very high in the three micelle Interestingly, the hydration number of the three F atoms decreased in the three systems according to the sequence TX-100>TX-5>TX-114.
Figure 8 Hydration number of atoms or benzene rings at different positions in meloxicam and celecoxib.
Note: See (Figure 4) for the label of each atom or benzene ring.
Number of Hydrogen Bonds: In this section, we further observe the hydrophilicity and hydrophobicity of two drug molecules by calculating the number of hydrogen bonds formed between polar atoms and H atoms at different positions in the drug molecule and water molecules, as shown in (Figure 9). The figure shows that the hydrogen bond numbers of O atoms at different positions in each drug molecule are very high, which corresponds to their hydration numbers. It is worth noting that the hydration number of H4 atom in meloxicam is the highest, and the hydrogen bond number is the lowest. The hydrogen bond number of three F atoms in Celecoxib is 0, indicating that the two drug molecules are relatively stable in each micelle. In addition, it can be seen from the figure that the number of hydrogen bonds of O4 atom in meloxicam is the largest in the three systems, and its value is about 1.5. The number of hydrogen bonds of H4, N1 and N2 atoms is close to 0. The hydrogen bond numbers of the O1 and O2 atoms of Celecoxib in the three systems are all greater than 0.5, while the N2 atom has the lowest hydrogen bond number, with a value of almost 0. It indicates that the interactions between two drug molecules and water in the three micelles are different. Overall, the interaction between two drug molecules and environmental water is relatively weak. This is mainly because meloxicam and Celecoxib are hydrophobic drugs, and their water solubility is poor. After dissolving in the micelles, except for individual polar O atoms forming hydrogen bonds with water molecules, most of the other polar atoms interact with the micelles, that is, the three TX micelles can improve the solubility of these two drug molecules.
Influence of Solubilization of Meloxicam and Celecoxib on Hydration Number and Hydrogen Bond Number of Micelles
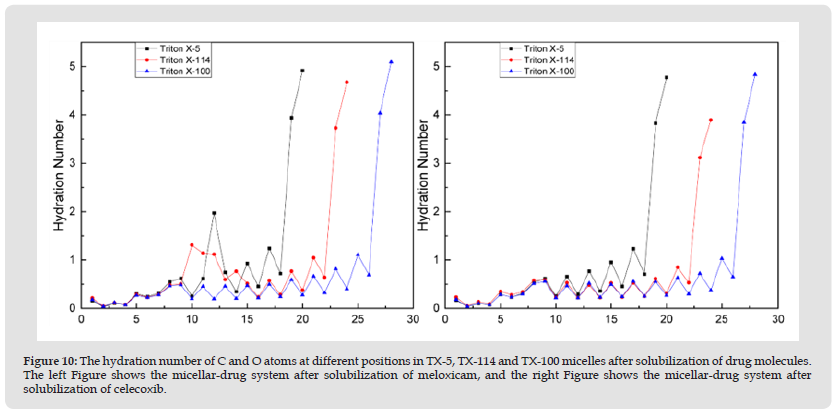
Hydration Number of Micelles: To investigate the effect of solubilization of two drug molecules on the hydration numbers of three micelles, we calculated the hydration numbers of C and O atoms at different positions in each micelle after solubilization of drug molecules, as shown in (Figure 10). The figure shows that meloxicam significantly increases the hydration number of ethylene oxide unit close to benzene ring in TX-5 and TX-114 micelles. Celecoxib resulted in a decrease in the hydration number of polar hydroxyl groups in both TX-114 and TX-100 micelles. This may be due to the different distribution positions of the two drug molecules in each micelle, which affects the contact range between the micelles and water. The hydration number of octyl chain in the three micelles solubilized with meloxicam and celecoxib was very low, and then the hydration number of benzene ring increased, and the hydration number of PEO chain in the three micelles solubilized with celecoxib showed a wavy trend. The hydration number of polar head hydroxyl groups was greater than 8 in all three systems solubilized with meloxicam, and greater than 6.9 in all systems solubilized with celecoxib. After solubilizing a single drug molecule, the interaction between the three TX micelles and water has not changed much, but more interaction with drug molecules, which further proves that the nanoscale TX micelles can be used as effective DDV of meloxicam and Celecoxib. At the same time, it also has certain reference significance for the formulation of other TX non ionic surfactant molecules and NSAIDs.
Figure 10 The hydration number of C and O atoms at different positions in TX-5, TX-114 and TX-100 micelles after solubilization of drug molecules. The left Figure shows the micellar-drug system after solubilization of meloxicam, and the right Figure shows the micellar-drug system after solubilization of celecoxib.
Note: See (Figure 12) for the atoms corresponding to each number.
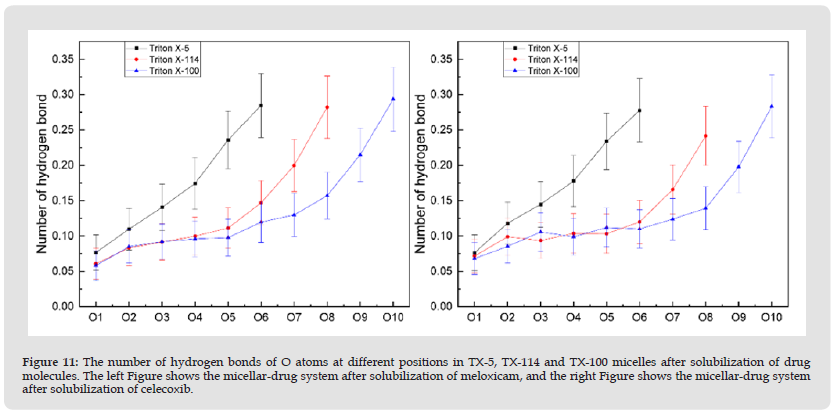
Number of Hydrogen Bonds in Micelles: In order to further explore the influence of the two drugs on the interaction between the micellar system and water, we used the number of hydrogen bonds formed between the O atom and the water molecule at different positions in each micelle. As can be seen from (Figures 11 & 12), Meloxicam slowed down the rise in the number of hydrogen bonds in the TX-114 micellar system, while celeoxib significantly reduced the number of hydrogen bonds in the TX-114 and TX-100 micellar systems, that is, after the drug molecules were dissolved in the micelle, they formed hydrogen bonds with water, which reduced the contact between O atoms and water molecules in different positions in the micelle.
Figure 11 The number of hydrogen bonds of O atoms at different positions in TX-5, TX-114 and TX-100 micelles after solubilization of drug molecules. The left Figure shows the micellar-drug system after solubilization of meloxicam, and the right Figure shows the micellar-drug system after solubilization of celecoxib.
Note: See (Figure 4) for atomic labels.
In this system, MD simulation was used to investigate the structural properties of TX-5, TX-114 and TX-100 micelles after solubing meloxicam and celecoxib. The simulation results show that the solubilization of celecoxib increases the ellipsoidal degree of TX-114 micelles significantly, and the total SASA of TX-5 micelles increases after solubilization of meloxicam and celecoxib, while the results of TX-114 and TX-100 micelles both decrease. In addition, the distribution of two drug molecules in each micelle showed that meloxicam was mostly distributed in the surface layer of the micelle compared with celecoxib. Importantly, the TX-100 micelle is the best for the encapsulation of two drug molecules. It should be noted that two drug molecules interact with three micelles through hydrophilicity and hydrophobicity. The simulation results of the interaction between drug molecules and water showed that meloxicam and celecoxib were both stable in the three micelles. This work provides a reference for pharmaceutical formulations based on Triton X micelles and NSAIDs.


