Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Eva María Molina Trinidad1,2*, Carmen Balderas Delgadillo3, Marco Antonio Becerril Flores1, José Alberto Ariza Ortega4, José Ramón Montejano Rodríguez5, Georgina Almaguer Vargas5, Laura Rosa Cornejo Roldán1, María Teresa Sosa Lozada1 and Daria Czeczuk6
Received: April 01, 2023; Published: May 04, 2023
*Corresponding author: Eva María Molina Trinidad, Área Académica de Medicina, Instituto de Ciencias de la Salud, Universidad Autonóma del Estado de Hidalgo, Circuito Ex-Hacienda La Concepción, Km 1.5. C.P. 42160. San Agustín Tlaxiaca, Hidalgo, Mexico
Departamento de Ingenieria y Tecnología, Farmacia, Facultad de Estudios Superiores Cuautitlán, Universidad Nacional Autónoma de México, Depto de Ingeniería y Tecnología (Biofarmacia), Cuautitlán Izcalli, Edo de Méx, México
DOI: 10.26717/BJSTR.2023.50.007917
Osteosarcoma is the name of a heterogeneous group of malignant spindle cell tumors that have immature bone production as a common feature. Sarcomas in this family range from those that can be cured with surgery alone to those that are highly lethal, even when treated with the most potent drugs. Osteosarcoma comprises a family of lesions with a considerable variety of features and histologic grades. Therefore, care for patients with osteosarcoma often takes place in a multidisciplinary cancer center where resources and staff provide appropriate care for patients with this type of cancer. Given the incidence in the pediatric and young population, documentary research of scientific journals and bibliographic types was carried out, based on the histology of osteosarcoma to update the information regarding this type of cancer and mention the importance of knowing this bone damage that can affect populations. susceptible to this disease. This review summarizes the pathogenesis and molecular aspects, clinical presentation, imaging, typified stages, histology, microscopy, treatment, chemotherapy, surgery, prognosis, and surveillance of osteosarcoma.
Keywords: Metastatic; Osteosarcoma; Pathogenesis; Chemotherapy; Surgery; Biopsy; Prognosis; Surveillance of Osteosarcoma
Abbreviations: MTP: Muramyl Tripeptide; CGH: Comparative Genome Hybridization; DNA: Deoxyriblonucleic Acid; CDKs: Cyclin Dependent Kinasa; GWAS: Genome Wide Association Study; G1: Phase in Which the Cell Prepares to Divide Cell; E2F: Transcription Factor Involved in the Cell Cycle; RB1: Gene involved in Cell Growth and Division; PMS Sarcoma: Pleomorphic Sarcoma; TP53: Tumoral Suppressor of Phosphoprotein; TAD: Transactivation Domain; PRD: Proline Rich Domain; DBD: DNA Binding Domain; OD: Tetramerization Domain; DR: Regulatory Domain; RecQ Helicase: Enzyme that Maintains the Integrity of the Genome; RECL4: Gen involved in and Repair; BLM: Blond´s Mutagenic Gene; WRN: Werner Syndrome Gen; S: Synthesis Phase in Cell Division; PET: Positron Emission Tomography; SALP: Serum Alkaline Phosphatase; CDKN2A: Gene involved in Differentiation, Division and Apoptosis the Cell Cycle; WHO: World Health Organization
Osteosarcoma refers to a bone tumor and the term was introduced by John Abernathy in 1804 referring to the fleshy growth [1]. In 2009, Ottaviane G. et al reported that osteosarcoma is an aggressive malignancy that arises from primitive transformed cells of mesenchymal origin and produces malignant osteoid, which are the most common representation of primary bone cancer [2,3]. Osteosarcoma represents a heterogeneous group of malignant spindle cell tumors that have as a common feature the production of immature or osteoid bone. On the other hand, bone histology indicates the degree of metastatic development. Sarcomas can be removed by surgery and can also be fatal. A familial genetic predisposition to osteosarcoma has been reported. It is believed that this disease is manifested by a mutation due to a deletion on chromosome 13 of the long arm q of band 14, where the retinoblastoma gene associated with the development of osteosarcoma is inactivated. Other diseases related to osteosarcoma are bone dysplasia, Paget's disease, fibrous dysplasia, enchondromatosis, and hereditary multiple exostosis. The germline TP53 mutation present in Li-Fraumeni syndrome is said to be a predisposing factor for the development of osteosarcoma. The autosomal recessive association of congenital bone defects, hair and skin dysplasia, hypogonadism, and cataracts present in Rothmund-Thomson syndrome is also related to osteosarcoma. Other conventionally variant alterations are the presence of osteoblastomas, chondroblastoma and fibro blastomas. Osteosarcoma can be multifocal, small cell, well-differentiated intraosseous, intracortical, periosteal, parosteal, high-grade superficial and extraosseous. Regarding the treatment of osteosarcoma, complete radical surgical resection is the treatment of choice.
Causative agents such as chemicals, viruses, radiation and miscellaneous are factors that induce osteosarcoma [4]. Chemical agents that cause genetic alterations and induce osteosarcoma are beryllium compounds and methylcholanthrene. Standard therapy includes a combination of limb-sparing orthopedic surgery when possible (or amputation in some cases) and a pharmaceutical combination of methotrexate with leucovorin, intra-arterial cisplatin, adriamycin and ifosfamide, and muramyl tripeptide (MTP). Rotationplasty is also another surgical technique that can be used. Ifosfamide can be used as adjuvant therapy if necrosis is low. Blood transfusions and Epogen medications help with anemia [5-7]. Treatment can be individualized by considering the genetics and biological variability of the individual patient [8-10]. It has been reported that more than 75% of osteosarcoma cases occur in subjects younger than 25 years [11,12]. Those that occur in adults are more likely to be secondary sarcomas; particularly those originating from Paget's disease, heart attacks, and previously irradiated tissues. In general, osteosarcomas are considered to be slightly more common in men, perhaps due to a longer period of skeletal growth compared with women [13]. An exception to this trend is periosteal osteosarcoma, which is more common in women [14]. No ethnic predilection has been observed in this type of cancer, but has been reported as Ewing's sarcoma and depends on age and type of tumor [15-17]. Germline mutation in the gene can also occur and characterize patients with a family history of Li-Fraumeni syndrome [18].
Etiopathogenesis
Genetic alterations define the progression from a normal cell to a cancerous one. Several molecular alterations occur in osteosarcoma; the most studied are the alterations that annul the normal function of p53 and Rb (product of the retinoblastoma susceptibility locus). Both lesions affect cell cycle regulation and proliferation control [18,19].
Genetic Instability in Osteosarcoma
Genetic aberrations have been investigated at the chromosomal and genetic level. Conventional cytogenetic analysis, molecular analysis of allelic imbalance, comparative genomic hybridization (CGH) and array-based CGH have identified many regions of loss, amplification and rearrangement in osteosarcoma. Instability can range from accelerated mutation rates, arising from defects in DNA repair, to major changes in DNA content resulting from errors in chromosome division during mitosis. Segregation errors lead to abnormal chromosome content, which is usually two to three times the content of normal diploid cells. Despite the complex pattern of genetic changes in osteosarcomas, certain chromosomal regions appear to be affected more often than others and may highlight the location of genes involved in the development and progression of these tumors [19]. Conventional cytogenetic techniques have shown that the most frequent chromosomal abnormalities are gain of 1 and loss of 9, 10, 13 and 17.
Partial or complete loss of the long arm of chromosome 6 was also observed. Common chromosomal rearrangements were found at 1p11–;13, 1q10–;12, 1q11, 1q21–;22, 4q27–;33, 6p23–;25, 7p13–;22, 7q11–;36, 11p10–;5, 11p14–;15, 12p13, 14p11–;13, 15p11–;13, 17p12–;13, 19q13, and 22q11–;13. Comparative genomic hybridization (CGH) has identified additional chromosomal abnormalities in osteosarcoma, including amplifications at 1q21, 3q26, 6p, 6p12.1, 6p12–;21, 8p, 8q12.21.3, 8q22–;q23, 8q24.2, 12q12–;13, 12q13–;q14, 14q24–;qter, 17p11–;12, Xp11.2–;21 and Xq12. Furthermore, loss of DNA sequence has also been observed at 2q, 6p, 8p, 10p and 17p13 [20-22]. These data suggest that genomic instability is an important feature of osteosarcoma, implicating pathways that normally maintain genomic enzymatic integrity with CDKs. [23,24]. Recent studies demonstrate the existence of CDK inhibitors (CDKI), in addition to the alteration of p16 NK as a tumor suppressor, which is especially important in some neoplasms. Alteration of p16 1NK4 appears to target characteristics that favor growth in culture. The p16 1NK4 tumor suppressor is the prototype of a family of related CDKIs. Recent research has established that p15 1NK4B, p18 and p19 can bind to and inhibit CDK4 and CDK6 and act as CDKIs with similar properties to p16 1NK4. This functional similarity is reflected in the structure of the protein; all four contain highly conserved ankyrin domains. Growth detection of cells treated with transforming growth factor-D is associated with increased accumulation of p15 1NK4B but not p16 1NK4, suggesting that p15 1NK4B may have an important role in regulating proliferation. Since all of these CDKIs block entry into S phase and the initiation of DNA synthesis and proliferation, they are therefore all candidate tumor suppressor genes [25].
Osteosarcomas, like the vast majority of cancers, are still unknown, since it is very broad and heterogeneous, hence there are numerous divergences in the literature regarding the genetics of osteosarcoma. Despite this, there are what we call risk factors that can lead to a greater predisposition to developing osteosarcoma [26]. These include sex, age, race, growth, as well as previous radiotherapies carried out as a treatment for other cancers, certain bone diseases such as Paget's disease, and hereditary multiple osteochondromas as pseudotumoral bone lesions [27]. Among the risk factors are also genetic risk factors, which cause an increasing predisposition to the development of osteosarcoma in certain families. In fact, both tumor suppressor genes, oncogenes and genes encoding growth factors have been found to be possible inducers of the development of osteosarcomas, although they are not specific for it [28,29]. Finally, it is worth highlighting the syndromes related to hereditary cancer, which are characterized by the fact that they can give rise to several types of cancer, among which is osteosarcoma [30-34]. These syndromes are hereditary retinoblastoma, Li-Fraumeni syndrome, Rothmund-Thomson syndrome and others such as Bloom syndrome and Werner syndrome or Diamond-Blackfan anemia [34-39]. The last three have been related to an increased risk of suffering from osteosarcoma, but paradoxically they are the least studied syndromes/risk factors as causes of suffering from this bone cancer. On the other hand, hereditary retinoblastoma and Li-Fraumeni syndrome are the most studied diseases in relation to osteosarcomas [40-52].
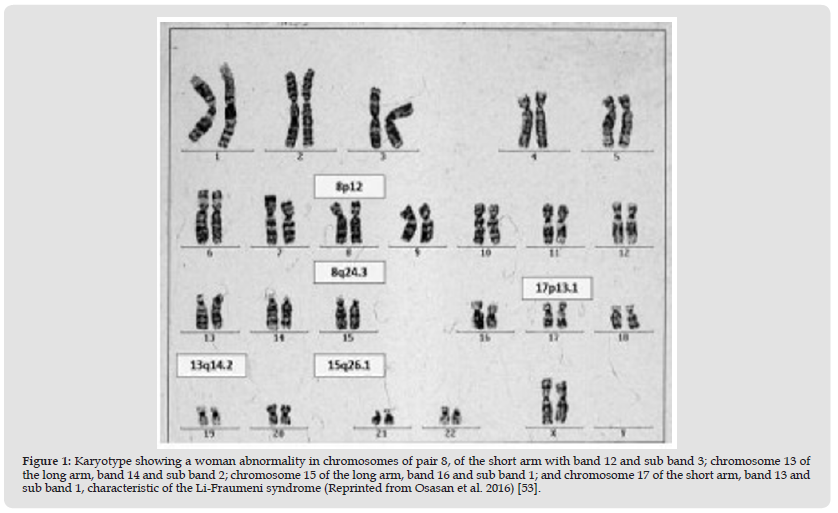
In Figure 1 we find a female karyotype (46XX), in which the different chromosome bands affected by the syndromes that are risk factors for the development of osteosarcoma are represented. On chromosome 8, specifically on the long arm, band 24 and subband 6, we would have Rothmund Thomson Syndrome. On chromosome 13, long arm, band 14 and subband 2 refer to the mutation for retinoblastoma. Chromosome 15, long arm, band 26, and subband 1 refer to Wener syndrome. And, finally, on chromosome 17, short arm, band 13 and sub band 1 refers to the Li-Fraumeni syndrome [52,53]. The figure shows the karyotype of an affected woman, reported by Osasan-Stephen et al.
Figure 1 Karyotype showing a woman abnormality in chromosomes of pair 8, of the short arm with band 12 and sub band 3; chromosome 13 of the long arm, band 14 and sub band 2; chromosome 15 of the long arm, band 16 and sub band 1; and chromosome 17 of the short arm, band 13 and sub band 1, characteristic of the Li-Fraumeni syndrome (Reprinted from Osasan et al. 2016) [53].
Types of the Osteosarcoma
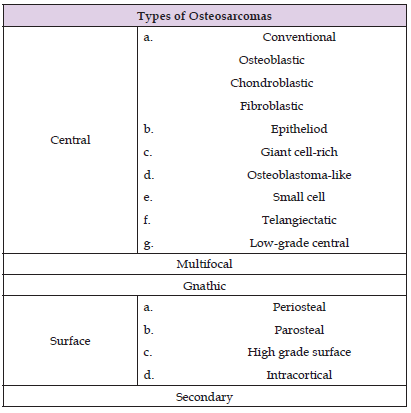
Most osteosarcomas are detected in the center of the bone marrow cavity of the long bone and may involve the periosteum, cortex, soft tissue or bony lesions. These tumors present variable amounts of osteoid with fibrous tissues and cartilage. According to the percentage of detection of the tumor, it can be considered as an osteoblast, chondroblast or fibroblast and the cell prevalence ranges from 50 to 80%, from 5 to 25% and from 7 to 25% respectively. Histological grades range from low, medium and high (grade 1, 2, and 3), identified by the tumor area of cellular degeneration and the highest mitotic rate. The following table reported by Xin Zhao et al. shows the different types of osteosarcomas (Table 1). It is said that well-differentiated osteosarcomas, as well as osteoblasts and chondroblasts, do not respond to chemotherapy treatments. Also, patients with this tumor can overcome metastasis, but it depends on the dominance of the cell type [54].
Table 1: Types of the osteosarcoma: central, multifocal, gnathic, of surface and secondary reported by Xin Zhao, et al. [54].
Risk Factors
Sex: In reference to sex, there is a predominance of males over females in a ratio of 1.5 to 1. On the other hand, the female sex that develops an osteosarcoma has a peak incidence at younger ages than the male sex and according to the bibliography, this is due to the fact that the female sex tends to present the so-called growth spurts before the male sex [55].
Age: According to the literature, the greatest risk of developing osteosarcoma occurs at very early ages, normally between 15 and 20 years of age, with a high number of cases in children and adolescents [9]. These data refer to the first incidence peak of this type of cancer and it is closely linked to the growth of children. In addition, it should be noted that in the ages between 60-65 to 80 years of age, there is a second incidence peak that in most cases is usually related to another previous and long-term bone disease, such as It's Paget's disease [55-62].
Race: In reference to ethnicity, most of the articles in the bibliography comments that there is no significant prevalence among the different races. But it is true that some bibliographical references indicate that there is a prevalent diagnosis in blacks and Hispanics compared to whites with respectively 6.8, 6.5 and 4.5 cases per year. In studies of osteosarcoma in children and adolescents it has been associated with the whole genome (GWAS), where two risk alleles were identified in subjects of European descent. Metastasis-related single nucleotide polymorphism was identified in subjects of European, African, and Brazilian descent with the disease due to alteration in telomeres in some cases [62,63].
Radiation as Treatment for Other Cancers: Ionizing radiation, such as radiotherapy treatments used to be a cure for the other types of cancer, can be a risk factor for the subsequent development of osteosarcoma around the area that was treated for the other cancer [63]. In general terms, ionizing radiation as a treatment in patients with osteosarcoma is limited to the primary use of chemotherapy administered intra-arterially and at the same time radiation is given where the amount of radiation exposed must be controlled [64,65].
Hereditary Retinoblastoma: Retinoblastoma is caused by the RB1 mutation, which is the cause of retinoblastoma, and it occurs on chromosome 13, long arm, band 14 and subband 236. That is, there is a homozygous loss of the retinoblastoma gene or this gene may be altered. As a consequence of this loss or alteration, a phosphoprotein phosphatase, pRb, is produced, which functions as a tumor suppressor of cell growth, stopping the cycle between the G1 phase and the synthesis phase (S). During the G1 phase of the cell cycle, pRb binds to transcription factors belonging to the E2F family, specifically E2F1, E2F2 and E2F3, which causes the cell cycle to be altered. Most osteosarcomas produced as a second extraocular tumor are due to this same mutation. Histological studies indicate its identification in photoreceptor cells. This disease is considered to be of the autosomal dominant type, where both alleles of the RB1 gene are mutated, inactivating the pRb, producing alterations in the cell cycle [23,66-68]. Germline RB1 carriers in whom Rb develops are also at risk of sarcoma pleomorphic (PMS) reported to be 20% at 30 years and 50% at 50 years. The spectrum of PMS includes osteosarcoma, soft tissue sarcoma, melanoma, and epithelial carcinoma of the lung and bladder [67]. The figure 2 showed ultra-widefield photographs of retinoblastoma, reported by Guarache et al. [68].
Figure 2 A) Macroscopic histopathological section. The tumor occupying the eyeball and the dislocation of the crystalline lens are observed. B) Histopathological section where tumor cells invade the sclera (thin arrows) and form an extrascleral nodule (thick arrow) using hematoxylin/ eosin staining at 100X objective (Reprinted fron Guarache et al. 2007) [68].
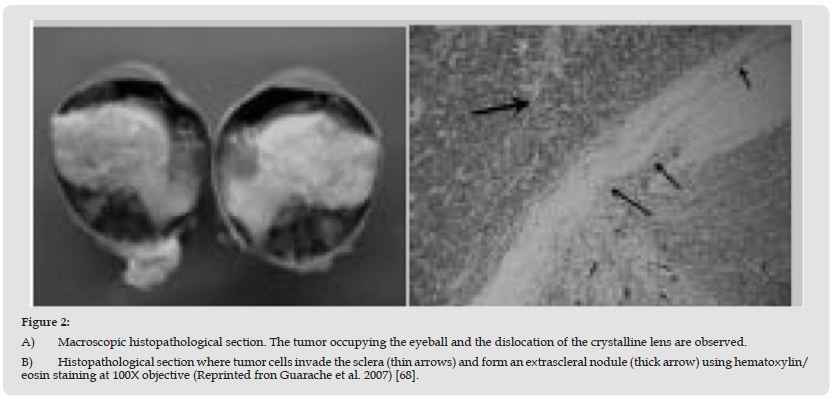
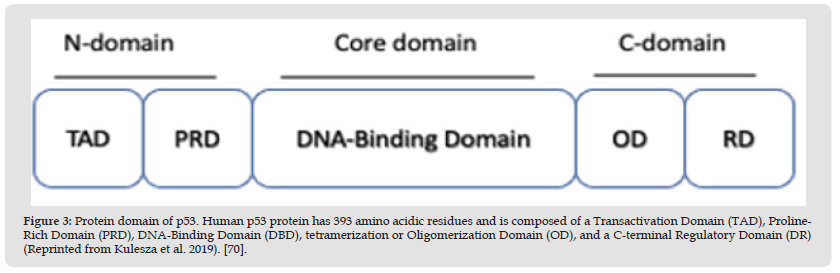
Figure 3 Protein domain of p53. Human p53 protein has 393 amino acidic residues and is composed of a Transactivation Domain (TAD), Proline- Rich Domain (PRD), DNA-Binding Domain (DBD), tetramerization or Oligomerization Domain (OD), and a C-terminal Regulatory Domain (DR) (Reprinted from Kulesza et al. 2019). [70].
Li–Fraumeni Syndrome
Li-Fraumeni syndrome presents the mutation in chromosome 17, specifically in the short arm, band 13 and sub-band 136. This syndrome consists of a familial predisposition to cancer, not only to osteosarcomas, but can also be associated with brain cancer [23,68,69]. Associations between tumor suppressor TP53 genotype and clinical tumor phenotype according to TP53 status have been reported. According to investigations, there is no association with specific mutation classes or TP53. The nuclear phosphoprotein p53 was first discovered in 1979 as a transcriptional factor associated with the SV40 large T antigen in virally transformed cancer cells, considered a proto-oncogene. The p53 protein functions as a tetramer, each monomer consisting of an N-terminal transactivation domain (TAD), a proline-rich domain (PRD), a core DNA-binding domain (DBD), a tetramerization domain (OD) and a C-terminal regulatory domain (DR). See (Figure 3), reported by Kulesza M et al. [70].
Rothmund-Thomson Syndrome
An autosomal recessive syndrome due to a mutation in the RECQL4 gene, it conveys a predisposition to osteosarcoma, as well as a characteristic infantile rash, dysplastic bone structures, alopecia, premature cataracts and chronic gastrointestinal distress. Rothmund-Thomson syndrome is characterized by an autosomal recessive genodermatosis that presents in children and is manifested by a characteristic facial rash known as poikiloderma, short stature, sparse hair on the scalp, few or absent eyelashes and/or eyebrows, juvenile cataracts, skeletal abnormalities, radial ray defects, premature aging, and predisposition to osteosarcoma. According to cytogenetic studies and chromosomal instability of the syndrome, it has been observed that the chromosomal instability of RecQ helicase mutant cells is a secondary manifestation of primary defects in replication and repair functions. On the other hand, it is difficult to establish a clinical diagnosis due to the description of chromosomal instability in patients with this disease before the RECQL4 molecular test became available; the variability in the procedure applied as spontaneous or induced chromosome instability and the variability in the cells used for the test as lymphocytes, fibroblasts and lymphoblastoid cell lines [71,72]. The figure 4 shows the telangiectasis of this syndrome, reported by Hernández-Bitor et al. [73].
Figure 4 Clinical features of RTS syndrome. Poikiloderma, (Figure 3). Telangiectasias on the anterior face of the neck and infraclavicular regions (Reprinted from Hernández et al. 1979) [73].

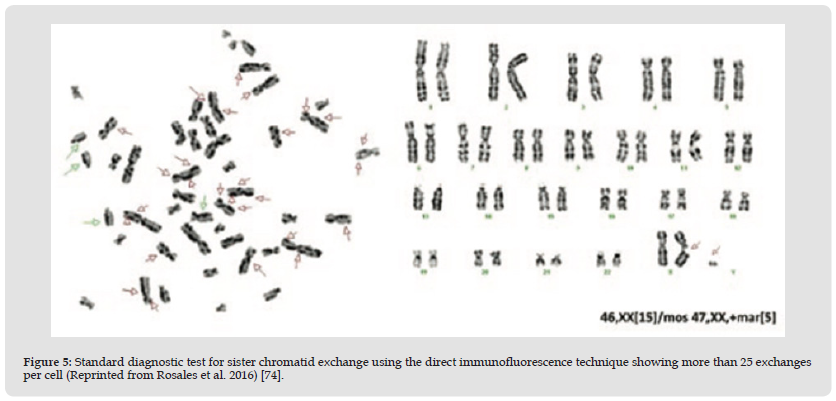
Figure 5 Standard diagnostic test for sister chromatid exchange using the direct immunofluorescence technique showing more than 25 exchanges per cell (Reprinted from Rosales et al. 2016) [74].
Bloom Syndrome
Autosomal recessive disorder caused by mutations in the BLM gene, a gene responsible for maintaining DNA stability during replication. In addition to a predisposition to osteosarcoma and other cancers, these patients may also have UV-induced rashes, short stature, and scant subcutaneous fat. Bloom syndrome is a rare autosomal recessive disease characterized by photosensitivity, facial telangiectasias, short stature, varying degrees of immunological disorders, and a high predisposition to various malignancies, associated with chromosomal instability. It was described in 1954 by David Bloom and is associated with diabetes mellitus, hypogonadism, infertility and ocular disorders. Few cases have currently been reported worldwide, but they have been reported in the Ashkenazi Jewish population. The characterization of this syndrome is done by identifying sister chromatids as a standard test. Differential diagnoses include Rothmund-Thomson syndrome, erythropoietic protoporphyria and Cockayne syndrome. A standard sister chromatid exchange diagnostic test for diagnosing Bloom Syndrome using the direct immunofluorescence technique is shown in the figure 5 reported by Rosales-Solís et al. [70,74].
Werner Syndrome
An autosomal recessive disorder, also known as adult progeria, is characterized by premature aging, bilateral cataracts, osteoporosis, short stature, scleroderma-like skin changes and a predilection for osteosarcoma. A defective WRN gene is responsible [66]. It is an autosomal recessive disorder characterized by accelerated aging in which patients generally develop normally until they reach adolescence. The first sign is the growth of the subject and a short stature in adulthood. In the early third decade of life, patients begin to develop an aged appearance that includes skin atrophy, loss of subcutaneous fat, and graying and hair loss, and the presence of bilateral cataracts requiring surgery seen at the age of 20 years [75].
Hereditary Retinoblastoma
Retinoblastoma is caused by the RB1 mutation, which is the cause of retinoblastoma and it occurs on chromosome 13, long arm, band 14 and subband 236. It is a homozygous loss of the retinoblastoma gene or this gene may be altered. As a consequence of this loss or alteration, a phosphoprotein phosphatase, pRb, is produced, which functions as a tumor suppressor of cell growth, stopping the cycle between the G1 phase and the synthesis phase (S). During the G1 phase of the cell cycle, pRb binds to transcription factors belonging to the E2F family, specifically E2F1, E2F2 and E2F3, which causes the cell cycle to be altered. Most osteosarcomas produced as a second extraocular tumor are due to this same mutation [23,66-67].
Signs and Symptoms
There is a history of pain, palpable mass when there is significant growth and limitation of mobility of the adjacent joint. Patients generally manifest a history of trauma that draws attention to the injury in question. The prevalence of pathological fracture both at the time of diagnosis and during the administration of preoperative chemotherapy is between 5 and 10%. Late signs of the disease include weight loss, fever, malaise, prostration and evidence of metastatic disease, which in sarcomas is usually pulmonary. In the affected area, the volume increase becomes so significant that it causes the appearance of striae and collateral venous network, gradually limiting to extreme degrees the mobility of the affected limb and even of the patient himself. Ulceration of the skin may occur, this happens when it is, most of the time, in the setting and location of some previous surgical procedure, generally biopsy [13]. Regarding osteosarcoma in the oral region, we find pain or paresthesia, rapid growth, inflammation and expansion of the cortical bone, facial asymmetry, nasal obstruction, displacement and mobility of neighboring teeth [76].
Diagnostic Method
1. Radiography
2. Blood tests: Some of the test results may be abnormal when osteosarcoma is present. For example, liver tests and the erythrocyte sedimentation rate (ESR) may be higher than normal.
3. Computed tomography.
4. Magnetic resonance.
5. Bone scan: A small amount of dye is injected into a vein. The whole body is analyzed. The dye is seen in areas where there may be cancer.
6. Positron emission tomography (PET) scan: For this test, radioactive sugar is injected into the bloodstream. Cancer cells use more sugar than normal cells, so the sugar is concentrated in the cancer cells. A special camera is used to see where radioactive sugar accumulates in the body. A PET scan can sometimes find cancer cells in different areas of the body, even if they can't be seen on other scans. This analysis is usually done in conjunction with a CT scan.
7. Tumor biopsy: A sample of the tumor is removed and checked under a microscope to see if there are cancer cells. A biopsy is required to diagnose osteosarcoma. The biopsy can be done with a needle or through surgery [76,77].
Clinical and Imaging Characteristics
Conventional Osteosarcoma: Its usual location is in the metaphyseal region of the long bones, mainly the femur (distal), the tibia and the humerus (proximal). The radiological characteristics of conventional osteosarcoma include both medullary and cortical bone destruction, aggressive periosteal reaction, soft tissue mass and the presence of tumor bone within the destructive lesion or in its periphery, as well as within the soft tissue mass. It is important to mention that there is no single radiological image that is pathognomonic for osteosarcoma. From the histopathological point of view, conventional osteosarcoma reveals very pleomorphic spindle tumor cells and other polyhedral osteoid-producing cells [78].
Mandibular Osteosarcoma: Generally, this variety of osteosarcoma occurs in older patients than those in whom the neoplasm is located in conventional sites and 50% of them present chondroblastic differentiation and osteoid production may be minimal and difficult to recognize. Approximately half of the patients have lesions classified as grade 2 or intermediate (Broders scale). Radiologically, they usually present radiolucent areas or a combination of these with radiodense areas [77,79]. The initial and most frequent clinical presentation is pain in the affected area, which may or may not be associated with an increase in the volume of soft tissues. Occasionally it can present as a pathological fracture, although this constitutes a minority of cases. The average duration of symptoms until the time of diagnosis is variable, but it is frequently three months and can last up to six or more months, although this is uncommon [78]. The characteristics of the pain are as follows: it is localized to the site of the injury, constant and progressive, in the initial phases it can be intermittent and respond to treatment with analgesics and anti-inflammatories, which will be insufficient over time. As the disease progresses, alterations in the angles of movement of the affected joint, limitation of function that progresses to disabling, increase in volume, visible and palpable tumor can be detected [79]. The initial study in the first contact is the evaluation with simple radiography of the affected area, which allows evaluating the bone architecture and soft tissues.
The study must be a comparative X-ray of the affected area, including at least two longitudinal planes of the area to be evaluated. 80% of osteosarcomas occur in tubular bones, between 50% to 75% occur in the knee followed by the shoulder and hip. The radiological pattern is variable, but is typically characterized by an ill-defined metaphyseal lesion, originating in the medullary space with new bone production, cortical rupture, periosteal reaction, and soft tissue tumor. Some characteristic images include Codman's triangle and the sunray image, due to the appearance of new bone formation in the surrounding soft tissue. Pathological fractures can occasionally be seen [79].
Osteosarcoma Staging
Staging is the process of determining how far a cancer has spread. Treatment and prognosis for osteosarcoma largely depend on the stage of the cancer when it was first diagnosed. The stage of an osteosarcoma is based on the results of physical exams, imaging tests and biopsies that have been done [76]. A staging system is a standard way the cancer care team uses to summarize the extent of the cancer. They often use a simpler system that divides osteosarcomas into 2 groups: localized and metastatic [80].
Localized Osteosarcoma: A localized osteosarcoma is seen only in the bone that started in tissues and possibly adjacent to the bone, such as muscle, tendon or fat. About 4 out of 5 osteosarcomas are believed to be localized where they are first found. But even when imaging tests don't show that the cancer has spread to distant areas, most patients are likely to have micro metastases (very small areas of cancer spread that cannot be detected with tests), so chemotherapy is an important part of treatment for most osteosarcomas. If it is not given, the cancer may be more likely to come back after surgery [79].
Metastatic Osteosarcoma: A metastatic osteosarcoma has clearly spread to other parts of the body, such as the lungs or to other bones that are not directly connected to the bone where the tumor started. It most often spreads to the lungs, but it can also spread to other bones, the brain or the other organs [17-18]. Approximately 1 in 5 patients with osteosarcoma have metastases at the time of diagnosis. These patients are more difficult to treat, but some can be cured if the metastases can be removed by surgery. The cure rate of these patients is markedly improved if chemotherapy is also given [79,80].
T-Categories:
1. T0: There is no evidence of a main (primary) tumor.
2. T1: The tumor is 8 cm (about 3 inches) across or less.
3. T2: The tumor is larger than 8 cm in diameter.
4. T3: The tumor has "jumped" to another site or sites on the same bone [23,75-78].
Bone Cancer Grades:
1. G1, G2: Low grade
2. G3, G4: High grade [23,77].
Histology
Histopathologic findings vary according to the particular subtype and can often be difficult to differentiate from fibro-osseous lesions such as fibrous histiocytoma, osteomyelitis, osteoradionecrosis, metastatic tumors, and other forms of sarcomas. However, common features of all subtypes are the presence of osteoid, normal or abnormal bone, closely associated with malignant connective tissue cells [18,80]. Osteosarcomas can be graded based on their cellularity, cellular atypia (cellular pleomorphism) and mitotic activity [81]. Histological diagnosis should be made only when the cell population is composed almost exclusively of small round cells that produce osteoid substance, at least in some areas. If this production is abundant, there are no difficulties in differential diagnosis with other small cell bone tumors (Ewing's sarcoma, lymphomas, primary neuroectodermal tumor). In contrast, when osteoid production is low or when the available biopsy is small, or in the form of frozen sections, the differential diagnosis can be difficult [82,83].
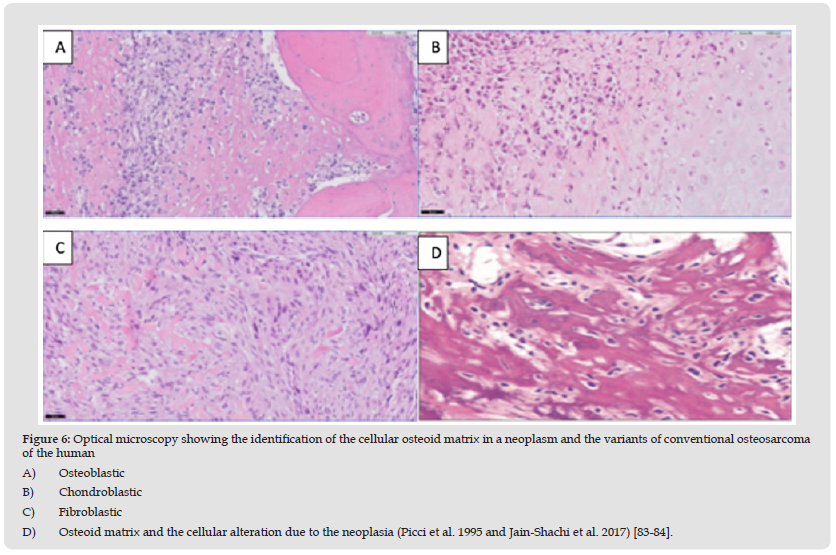
Osteosarcoma histology indicates the presence of invasive cells infiltrating the medullary canal and Hanvers' canals, surrounding pre-existing bone trabeculae. Soft tissue components tend to elevate the periosteum by depositing bone during this process. The presence of the tumor alludes to the histological diversity, for which these tumors are identified according to the function of the components of the neoplastic matrix that predominates in osteoblasts, fibroblasts and chondroblasts. In the former, they can be observed as large and pleomorphic cells with a polyhedral or spindle shape, presenting hyperchromatic nuclei with eosinophilic cytoplasm and based on the state of mineralization, the neoplastic bone can be eosinophilic or basophilic. Regarding the fibroblasts present in the tumor, cells with a spindle-shaped appearance are observed, with elongated nuclei and develop in the shape of a spike [83]. Figure 6 shows the cellular osteoid matrix in a neoplasm and the variants of conventional osteosarcoma of the human osteoblastic, chondroblastic, and fibroblastic reported by Jain-Shachi [84].
Figure 6 Optical microscopy showing the identification of the cellular osteoid matrix in a neoplasm and the variants of conventional osteosarcoma of the human A) Osteoblastic B) Chondroblastic C) Fibroblastic D) Osteoid matrix and the cellular alteration due to the neoplasia (Picci et al. 1995 and Jain-Shachi et al. 2017) [83-84].
Treatment
Localized Resectable Osteosarcoma: High-grade: Most osteosarcomas are high-grade, which means they are likely to grow and spread quickly if left untreated. The usual treatment for these cancers is as follows:
1. Biopsy to establish the diagnosis.
2. Chemotherapy, usually for about 10 weeks.
3. Surgery to remove the tumor, preferably by the same surgeon who did the biopsy. If cancer is found at the edge of the surgery sample (meaning some cancer may have been left behind), a second surgery may be done to try to remove any remaining cancer. Radiation therapy may also be given to the area.
4. More chemotherapy (for up to a year). If the initial chemotherapy kills most of the cancer cells, the same drugs are often given again after surgery. If the initial chemo didn't work well, different drugs may be tried (although not all doctors agree that switching drugs is necessary) [54,85].
Unresectable Localized Osteosarcoma: As with other osteosarcomas, a biopsy is first needed to establish the diagnosis. Chemotherapy is usually the first treatment for these types of cancer. If the tumor shrinks enough to become resectable, then it is removed with surgery. This is followed by more chemotherapy for up to a year. If the tumor still cannot be completely removed after chemotherapy, radiation therapy can often be used to try to keep the tumor under control and help relieve symptoms. More chemotherapy might be another option, either instead of or after radiation therapy. If the first chemotherapy regimen did not work very well, different chemotherapy drugs may be tried. Because these tumors can be difficult to treat, a clinical trial of newer treatments may be a good option in many cases [86].
These cancers have already spread to distant parts of the body when they are diagnosed. Most of the time they spread to the lungs. As with other osteosarcomas, a biopsy is first needed to establish the diagnosis. Chemotherapy is usually the first treatment for these types of cancer. If all tumors are thought to be resectable after chemotherapy, they are removed with surgery, sometimes in more than one operation. This is followed by more chemotherapy for up to a year. If some of the tumors remain unresectable after chemotherapy, radiation therapy can often be used to try to keep them under control and help relieve symptoms. More chemotherapy might be another option, either instead of or after radiation therapy. If the first chemotherapy regimen did not work very well, different chemotherapy drugs may be tried. Newer targeted drugs such as regorafenib (Stivarga), sorafenib (Nexavar), or cabozantinib (Cabometyx) may also be an option at some point. Because these tumors can be difficult to treat, clinical trials of newer treatments may be a good option in many cases [87].
Recurrent Osteosarcoma
Recurrent cancer means that the cancer has come back after treatment. It can come back locally (near where the first tumor was) or in other parts of the body. Most of the time, if the osteosarcoma comes back, it will be in the lungs. If possible, surgery to remove the tumors is an important part of treatment, as it offers the best chance of long-term survival. If the cancer comes back at the original site in an arm or leg after limb-sparing surgery, amputation of the limb may be recommended. Chemotherapy is also often part of the treatment for recurrent cancers. If the cancer is unresectable, chemotherapy may be used to try to shrink the tumor, which may then allow surgery to be done. If the cancer is resectable, chemotherapy may be given after surgery. For more advanced cancers, chemotherapy may be used to try to help relieve symptoms. Radiation therapy may also be part of the treatment. It can sometimes help control tumor growth and help relieve symptoms. If the cancer continues to grow, newer targeted drugs such as regorafenib (Stivarga), sorafenib (Nexavar), or cabozantinib (Cabometyx) may also be an option at some point. Because these tumors can be difficult to treat, clinical trials of newer treatments may be a good option [88].
Forecast
Numerous previous studies establish that the response to neoadjuvant chemotherapy, reflected in the percentage of tumor necrosis, represents the most important prognostic factor in patients with localized osteosarcoma. A good response is defined as a percentage of tumor necrosis ≥ 90% [89,90]. With the introduction of chemotherapy (CMT), before and after surgery in patients with localized disease, the 5-year survival rate rose from 20 to 70% [89,90]. The presence of lung metastases at the time of diagnosis clearly worsens survival at five years, reaching 21% in some cases. There is consensus in the literature that the degree of tumor necrosis less than 90%, induced by preoperative chemotherapy, is an important prognostic factor in relation to the survival of patients with primary OS [91]. On the other hand, there is controversy regarding other variables, such as: sex, location of the tumor, type of osteosarcoma and type of surgery, due to the variation in the methodology used, as well as the variability of prognostic factors considered, consider the classification [91,92].
It has been observed that when osteosarcoma is detected in a patient, it should be assessed by a multidisciplinary health team that includes oncology, traumatology and radiology. Knowing the mechanisms of chemoresistance of metastases due to loss of integrity of the apoptosis pathways, specifically the role of the enzymatic pathway, seems to be a new alternative in the treatment of osteosarcoma. A study in dogs indicates that one of the best-known prognostic factors for osteosarcoma is the measurement of serum alkaline phosphatase (SALP). Elevated levels of the enzyme and bone isoenzyme are associated with disease and survival time [93].
Currently, osteosarcoma has been defined as a type of cancer that is identified as a primary malignant tumor characterized by the identification of immature bones or the presence of osteoid tissue. Its incidence is low but it occurs in children, in young people and rarely in adults, being frequent in men and less frequently in women. Bone alteration occurs in long bones and tissues. This syndrome is associated with other genetic alterations related to the long and short arms of some pairs of chromosomes such as chromosomes 13, 15 and 17. In chromosome 13 there is an alteration in the long arm, in band 14 and sub-band two; in chromosome 15 there is a change in the long arm of band 16 and sub-band 1, and in chromosome 17 the change is observed in the short arm, band 13 and sub-band 1, reported by Czarneck et al. referring to the molecular biology of osteosarcoma and Kansara and Thomas regarding the pathogenesis of osteosarcoma, as well as González-Centeno referring to the etiopathogeny of osteosarcomas.
It is said that this cancer maintains genomic integrity with respect to cell cycle activating enzymes (CDK1), which act as inhibitors and are part of the recognition of suppressor tumors such as neoplasms related to cell proliferation, due to the alteration of the p16-inhibitor. Cyclin-dependent NK known as CDKN2A or p16. Its mechanism is based on interference in the S stage during the cell division process. On the other hand, the relationship that osteosarcoma has with other types of cancer is unknown, but its incidence depends on sex, age, race, and bone growth related to Piaget's disease. In relation to race, no preference has been identified, but it has been detected in the European, African and South American continents. It is said to be a hereditary cancer related to Li-Fraumeni, Rothmund-Thomson, Bloom, Werner syndrome and the manifestation of retinoblastoma, as mentioned by Osasan et al. relating this disease to osteogenic osteosarcoma. Osteosarcoma treatment is based on appropriate pharmacology after surgery and chemotherapy, as well as targeted radiotherapy. It has been reported that the diagnostic methods to identify this disease are related to obtaining X-rays, taking blood samples, application of computed tomography, nuclear magnetic resonance, bone scanning, positron emission tomography and obtaining biopsies.
Likewise, different mechanisms are available to categorize the different types of osteosarcomas and it is important to review the histology of this disease to identify specific subtypes to differentiate between lesions in bone fibers, presence of osteomyelitis, osteoradionecrosis, metastatic tumors or other forms of sarcoma, for example, in connective tissue, pleomorphism and mitotic activity can be observed. Finally, the treatment of osteosarcoma is based on the use of biopsies to establish a diagnosis, followed by treatment with chemotherapy and radiotherapy and surgery, depending on its type and location [94,95].
Osteosarcoma is classified as a bone tumor, based on its histology, according to the World Health Organization (WHO). It represents different subtypes based on its position and location, which is central or superficial. The diagnosis leads to the performance of a tissue biopsy to perform histological studies. According to the type of osteosarcoma and the stages of the tumor lesions (I to III) as the primary tumor, a diagnosis is given and later a treatment. The symptoms presented by an individual who is diagnosed with osteosarcoma can be evidenced by fever and anorexia. It should be said that the diagnostic methods begin with the location of the tumor by performing an X-ray on the patient. The life expectancy of a patient is high if the osteosarcoma is detected in time to be able to give an effective treatment.
None.
We thank the Autonomous University of the State of Hidalgo and the National Autonomous University of Mexico.


