Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Navneet Nagpal1, Parampal Kaur2, Nishant Kumar2, Sukash Kumar Gupta3, Manisha Arora1 and Prince Ahad Mir2*
Received: April 10, 2023; Published: April 28, 2023
*Corresponding author: Prince Ahad Mir, Assistant Professor, Department of Pharmacognosy and Phytochemistry, Khalsa College of Pharmacy, Amritsar, India
DOI: 10.26717/BJSTR.2023.50.007897
Beta-blocker atenolol is a well-known anti-hypertensive drug that has tendency to cause changes in the patient’s normal lipid profile.
Aim: This study aims to prevent this harmful impact of Atenolol utilising Glycine max seeds, a cost-effective, widely accessible nutraceutical that is more physiologically compatible than other choices. Materials and Method: Wistar rats were given a diet with 8% sodium chloride to induce hypertension and subsequently sacrificed. Glycine max powder was used to reduce atenolol’s side effects during the trial. Thirty rats were divided into five groups and tested over eight weeks. Rats’ blood pressure (BP) was measured with a tail-cuff NIBP monitor. Biochemical markers like TG, TC, HDL, LDL, VLDL, alanine transferase, aspartate transferase, lipid peroxide (LPO), catalase, and super oxide dismutase (SOD) were evaluated.
Results: Glycine max seeds may minimize the effects of Atenolol on the lipid profile by reducing blood cholesterol, increasing antioxidant levels, and maintaining liver function. When glycine max and Atenolol were administered together, there was a substantial decrease in TC, TG, LDL, VLDL, and HDL levels, SOD, catalase and increase in MDA, AST and ALT as compared to the normal control group (p≤0.05).
Conclusion: SBP (systolic blood pressure) and DBP (diastolic blood pressure) are lowered considerably compared to a control diet, however the amount depends on pre-treatment BP measurements and baseline comparison diet. Combining Atenolol with glycine max not only improves blood pressure but also modifies lipid profile. In rats with atenolol-induced hyperlipidemia, glycine max reduces oxidative stress and liver damage.
Keywords: Hypertension; Lipid Profile; Antioxidant Markers; Hepatic Profile; Atenolol; Soybean
Abbreviations: BP: Blood Pressure; ALT: Alanine Transaminase; TG: Triglyceride; SBP: Systolic Blood Pressure; TC: Total Cholesterol; DBP: Diastolic Blood Pressure; HDL: High Density Lipoprotein; CNS: Central Nervous System; LDL: Low Density Lipoprotein; CVS: Cardiac Vascular System; VLDL: Very Low- Density Lipoprotein; DDMDC: Diphenyl-Dimethyl-Dicarboxylate; SOD: Superoxide Dismutase; SGPT: Glutamic-Pyruvic Transaminase; LPO: Lipid Peroxidation; SGOT: Glutamic-Oxalacetic Transaminase; MDA: Malondialdehyde; EDTA: Ethylenediamine Tetraacetic Acid; AST: Aspartate Transaminase; GM: Glycine Max; NBT: Nitroblue Tetrazolium; NADH: Nicotinamide Adenine Dinucleotide; PMS: Phenazine Methosulfate
The soybean, or Glycine max (L.) Merr., is a subtropical plant that originates in Southeast Asia. At least five thousand years ago, soybeans were already being used as a major nutritional component in the diets of several Asian nations. Soybeans were fermented into more palatable forms during the Chou dynasty in China, resulting in foods like tempeh, miso, and tamari soy sauce [1]. The East Asian origin of this legume places it in the family Leguminosae. There is no definitive record of when or where the soybean was first domesticated, while claims that it originated in China are widely accepted [2]. Soy is rich in isoflavones, which regulate a wide range of physiological processes including tumour suppression, menopause relief, bone health, and ageing. It also has minerals and vitamins including calcium, potassium, and iron in addition to the saponins, vital amino acids, phytosterols, and other components. Soy isoflavone genistein increases the activity of antioxidant genes responsible for smoother skin and fewer wrinkles. Proteases aid in skin bleaching and hair reduction, two common cosmetic goals. As a result, many different types of skin care products have adopted this trend [3]. In adults, hypertension is defined as a rise in blood pressure against the arterial wall that is greater than 140 millimetres of mercury (mmHg) or 90 millimetres of mercury (mmHg) over the usual range (120/80 millimetres of mercury (mmHg) [4].
In a recent report, the World Health Organization (WHO) stated that hypertension is one of the most important risk factors for cardiovascular [5] diseases, which have affected more than 1 billion people around the world and act as the silent, unseen killer responsible for approximately 9.4 million deaths per year in the world [6]. Hypertension may be lethal for major bodily organs such as the brain and kidneys, and patients with the condition must make considerable lifestyle adjustments in addition to medication modifications in order to manage the condition. This illness has spread to epidemic proportions, necessitating the adoption of newer, more cost-effective, and better treatment procedures on a continuous basis [7]. For more than 40 years, atenolol has been regarded as the most cost-effective first-line antihypertensive medication available. It has been used therapeutically in low- and lower-middle-income nations to maintain normal blood pressure levels [8]. Inhibition of the positive inotropic and chronotropic activities of endogenous catecholamines such as isoproterenol, norepinephrine, and epinephrine results in the antihypertensive effect of the medication, which is a secondgeneration beta-1 cardio-selective adrenergic antagonist. The lower incidence of CNS effects in contrast to other beta-blockers may be attributed to the lower blood-brain barrier penetration and lower lipid solubility of Atenolol [9].
Over time, researchers have discovered that Atenolol has negative effects on the lipid profile, and it has been shown to raise the levels of TC, LDL, and TG, while decreasing the levels of HDL by some researchers. It has been reported that the lipoprotein effects of beta blockers (Atenolol) are caused by the inhibition of betaadrenergic activity, which then results in unopposed activation of alpha-adrenergic receptors. The activation of alpha-adrenergic receptors, on the other hand, causes a reduction in the activity of peripheral lipoprotein lipase, which, in turn, leads to a decrease in the catabolism of very low-density lipoprotein and triglycerides [10]. Abnormal lipid levels increase the likelihood of developing coronary heart disease by a large margin [11,12]. Health care practitioners are increasingly turning to alternatives to Atenolol for the treatment of a variety of cardiac problems. However, these options are more costly than Atenolol [13]. Clinical trials involving Atenolol and cholesterollowering drugs such as aspirin, simvastatin, and lisinopril have also been conducted to reduce the risk of several CVS diseases and deaths, although improvements in clinical risk factors were modest and did not reach statistical significance in some studies [14,15].
Further, previous studies suggested that long term induction of atenolol lead to cardiotoxicity in terms increase in oxidative stress in rats [16]. Furthermore, existing information suggested that long term treatment with atenolol used as an antihypertensive agent causes increase in serum liver enzymes in rabbit [17]. To reduce the danger of sickness and bad consequences, as well as to achieve excellent health, a large section of the world’s population favored natural therapies over or in combination with allopathic drugs [18]. In the field of nutrition, nutraceuticals are dietary supplements, foods, or portions of foods that provide medicinal or health advantages [19]. As a consequence, nutraceuticals are not always studied and controlled to the same level as pharmaceuticals, and using any of these items in conjunction with allopathic therapy may worsen or exacerbate the clinical symptoms and series outcomes, depending on the prescriptions being used [20]. While many drug scientists sought to create allo-polyherbal formulations as an excellent alternative to reduce unwanted effects or improve clinical conditions, this was not always successful, as seen in the following examples: The combination of Trigonella foenum-graecum (fenugreek), Momordica charantia (bitter gourd), Aegle marmelos (bael), and Glipizide increases the hypolipidemic impact while also reducing the cardiovascular risk factors linked with diabetes, according to the researchers. Hepatitis patients’ liver function improves when they take oil of Allium sativum in combination with diphenyl-dimethyl-dicarboxylate (DDMDC) [21,22]. Nutraceutical products could be labelled with a statement explaining their purported effect on the structure or function of the human body or their role in promoting general well-being under the Food Safety and Standards (Health Supplements, Nutraceuticals, Food for Special Dietary Use, Food for Special Medical Purpose, Functional Food, and Novel Food) Regulations, 2016, which came into effect in 2016 [23].
Because of its high protein content, soybean (Glycine max) is not only used as a traditional food component, but it is also used as a key ingredient in many processed foods, including dairy product alternatives. The soybean is a rich source of soy protein, as well as a range of vitamins and minerals, as well as isoflavones (plant estrogens) [24]. Natural soy protein is a high-quality source of L-arginine, which has been shown to help lower LDL cholesterol levels in the blood [25]. Soy isoflavones are also engaged in maintaining rhythmic cardiovascular health as well as minimizing the likelihood of developing cardiovascular disease. Both soy protein and isoflavones have been shown to lessen the risk of developing atherosclerosis [26]. Soy isoflavones in food to promote the preservation of good cardiovascular health seems to be promising, and more clinical studies in people with a specific objective are now being planned [27]. A meta-analysis of controlled clinical research came to the conclusion that soy protein substantially reduced total cholesterol, LDL cholesterol, and total triglyceride levels while having no effect on HDL cholesterol levels [28].
Curdle soyprotein has been used to protect liver damage and increase antioxidant activity in acetaminophen induced liver toxicity in rats [29]. Researchers also hypothesized that soybeans increased the transcription of the LDL-R gene in human liver cells, which resulted in increased catabolism or decreased production of intracellular cholesterol [30]. However it is unknown which bioactive component of soy protein is responsible for the change in lipid levels, oxidative stress and serum liver enzyme levels; it is known that soy protein has a lipid-lowering impact, although the mechanism of this action is not understood [31]. As a result, the objective of our investigation was to investigate the pre-clinical therapy of atenololinduced alterations in cholesterol levels, antioxidant parameters, and liver enzyme activity. It is anticipated that by establishing Glycine Max as a nutraceutical, we would be able to counteract the unfavorable impact that Atenolol has on the patient’s lipid profile. This study may serve as a demonstration of the greater efficacy of antihypertensive treatment using the medication Atenolol, which is less expensive and has less adverse effects on the lipid profile. Additionally, it may serve as a demonstration of the greater efficacy of treating hypertension with Atenolol. It is possible that people who originate from lowerand middle-income households may find this combination therapy to be a blessing in the future as a result of the cost-effectiveness of the treatment.
Material and Identification
The Glycine max seeds were purchased at a local market in Amritsar, where they were still fresh. The Postgraduate Department of Botany at Khalsa College in Amritsar recognised and validated the seeds based on their variety, chemical, and phytochemical makeup (Ref. No. KC/SS/1215/BD) and other characteristics. Jackson Pharmaceutical Industries Pvt Ltd, Amritsar, provided us with a gift sample of atenolol hydrochloride for testing purposes. AST kit (cat no. 120204; ERBA India), ALT kit (cat no. 120207; ERBA; India) were purchased from local vendors. Various pre-formulation experiments were used to identify potential drug ingredients. Everything that was employed in this experiment was of analytical grade, including the substances themselves.
Methods
Preparation of Glycine Max Powder: Glycine max seeds (moisture content 9.0 percent v/w) were pulverized in a laboratory grinder using fresh and regulated dry Glycine max seeds. After that, the crushed powder was passed through filter number 44. The moisture content of the manufactured powder was determined using an infrared moisture balance, and the powder was then kept under regulated moisture and temperature settings.
Ethical Clearance of Animals, Housing, and Diet: A total of 36 Albino Wistar rats (weighing around 250 g) were obtained from IIIM, Jammu, Jammu and Kashmir, India (Register No. of breeder-C7/ CPCSEA/99). In accordance with IAEC/KCP/2015, the Institutional Ethical Committee authorized the research (Approved protocol number 0016 of Letter Ref. No. IAEC/KCP/2015). The experimental procedure was developed and carried out at the Khalsa College of Pharmacy in Amritsar’s animal house facility in accordance with the standards of the Central Pharmaceutical Control and Standards Organization (CPCSEA) in New Delhi. A 12-hour light/dark cycle was utilised to house adult male and female rats in separate plastic cages at 222 °C and relative humidity 30 percent to 50% on a 12-hour light/ dark cycle. The animals were given a two-week acclimation period prior to being released [32]. The rats were given unrestricted access to the filtered water ad labium as well as to their typical rat chow diet, which was provided. The animals were provided with a clean cage on a regular basis starting on the first day of the acclimatization phase, which lasted 14 days. The rats’ body temperatures were checked on a regular basis and kept between 37.5 and 37.8 degrees Celsius. In accordance with the National Institutes of Health Guide for the care and use of laboratory animals, National Academies Press, eighth edition, the animals were provided with humane treatment, and the experimental method was meticulously carried out in order to prevent any stressful situations [33xperimental Study Design and Treatment: The saltinduced hypertension model was used to create hypertension in an experimental setting. In this method, the rats were fed a high-sodium rat chow diet to keep them healthy. To make a high sodium diet for rats, 8 percent NaCl was mixed into rat chow (8 percent NaCl diet), which was then fed to the rats [34]. After two weeks of acclimation, the animals were separated into five groups of six animals each (n=6 in each group).
• Group I received normal saline and rat food and served as
control.
• Group II received 8% sodium chloride (NaCl) and served as
toxic control.
• Group III received high sodium rat food and an
antihypertensive drug Atenolol (10 mg/kg bw).
• Group IV received high sodium rat food + Atenolol (10 mg/kg
bw) and glycine max (200 mg/kg bw) in combination
• Group V was considered nutraceutical control (200 mg/kg
bw).
Wistar rats from various groups were subjected to the abovementioned
daily food routine for an eight-week period [35].
The Following BP Parameters are Measured: When measuring blood pressure, we utilised an automated computerised tail-cuff noninvasive blood pressure (NIBP) monitor system for rats, model no. LE 5001 (Panlab, Harvard Apparatus, Spain), which was operated in accordance with the manufacturer’s instructions and published literature [36].
Estimation of LPO, Catalase and SOD in Blood Serum: LPO was estimated as per the method of Draper and Hadley with slight modification in which 2 mL of blood were centrifuged at 1000 RPM for 10 minutes at 4 °C to separate the serum. Instantaneous MDA assays were performed on serum samples. Serum samples were analyzed for their levels of malondialdehyde (MDA) using the thiobarbituric acid reaction technique. Absorption at 532 nm was compared to a reference curve of MDA equivalents formed by the acid-catalyzed hydrolysis of 1,1,3,3-tetramethoxypropane, and the concentration of thiobarbituric acid reactive compounds was calculated. U mol/l was used to represent MDA concentrations [37,38].
Estimation of Sod in Blood Serum: Superoxide dismutase (SOD) was determined using the technique of Kakkar et al (1984). The serum were homogenised at a concentration of 50 mg/mL in ice-cold sodium pyrophosphate buffer (pH 8.3); 200 L of this homogenate was utilised for the experiment. At 560 nm, the inhibition of NBT’s conversion to a blue-colored chromogen by SOD in the presence of PMS and NADH was measured. One unit of enzyme activity was defined as the enzyme concentration necessary to reduce the absorbance at 560 nm of chromogen synthesis by 50% in 1 minute under test conditions, and was represented as SOD/min/mg protein [39,40].
Estimation of Catalase in Blood Serum: 100 mL of 0.01M acetate buffer (pH 4.5) were put to a 250mL beaker and kept in a 37 °C water bath for 15 minutes. A 1 mL aliquot was obtained as a blank sample. Then, 30 mL (6.12 g/L) of H2O2 was added to the beaker. A 1 mL sample was obtained after 5 minutes to determine the initial H2O2 concentration. The reaction was initiated by adding 20 mL of a catalase solution (2,950 U, 5,900 U or 29,500 U). 1 mL aliquots were extracted every 5 minutes and submerged in boiling water for 3 minutes. The sample was then placed in a 1.5-mL quartz cuvette, and the remaining H2O2 concentration was measured at 240 nm. The cumulative response time was thirty minutes [41,42]. Estimation of Level of AST and ALT in Blood Serum: The serum samples were examined for liver (SGOT and SGPT) marker enzymes using commercial colorimetric assay kits, following the manufacturer’s protocol.
Measurement of a Lipoprotein Panel: After fasting for 12 hours, rats were sedated with diethyl ether and blood samples were obtained from the retro-orbital sinus of each rat. Fresh blood samples (2 mL) were collected and centrifuged at 4000 rpm for 10 minutes to separate the serum, before being analysed. In order to conduct the study, the serum was collected into simple caped tubes and refrigerated at -20 °C until it was used for analysis [43]. This study used a wet reagent diagnostic kit to detect the serum concentrations of biochemical analyses such as total cholesterol (TG), total triacylglycerol (TC), HDL, LDL, and VLDL levels, and a UV-Vis spectrophotometer (Shimadzu UV-1200) to quantify their absorbance [44]. The test is based on an enzyme-driven reaction that measures both cholesterol esters and free cholesterol in a single reaction tube. The procedure of determination was carried out in accordance with the instructions provided by the kit maker. The data were reported as a mean standard deviation (mean standard deviation). Microsoft Excel 2007 was used to conduct the statistical analysis, which consisted of one-way ANOVA.
As the diastolic blood pressure was measured after 04 and 08 weeks of therapy, it was discovered that Atenolol had antihypertensive effect in the albino Wistar rats when compared to the normal control group. The combination of a soybean diet with Atenolol demonstrated only a very mild and marginally significant antihypertensive effect (Table 1). Figure 1 depicts a visual representation of Atenolol’s antihypertensive action against hypertension. The effects of various treatment groups on their lipid profiles (TC, TG, LDL, VLDL, and HDL levels) after 04 and 08 weeks of therapy are shown in Table 2 and graphically illustrated in Figures 2-5, respectively.
Table 1. Effect of Atenolol and Glycine max on diastolic blood pressure of different treatment groups after 04 and 08 weeks treatment.
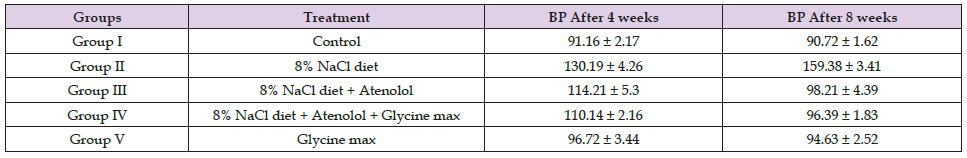
Note: Data values of BP are in mmHg and represented as Mean ± SD; n=3
The effects of various treatment groups on their lipid profiles (TC, TG, LDL, VLDL, and HDL levels) after 04 and 08 weeks of therapy are shown in table 2 and graphically illustrated in figures 2-5, respectively.
Note: Values of TC are in mg/dl and represented in mean ± SD.
(a=p<0.05 vs Atenolol + GM treatment, b=p<0.05 vs GM treatment, c=p<0.05 vs Atenolol treatment).
Table 2. Effect of Atenolol and Glycine max on lipid profile of different treatment groups after 04- and 08-weeks treatment.

Note: Data values of lipid profiles are in mg/dl and represented as Mean ± SD; n=3F
Note: Values of TG are in mg/dl and represented in mean ± SD.
(a=p<0.05 vs Atenolol + GM treatment, b=p<0.05 vs GM treatment, c=p<0.05 vs Atenolol treatment).
Note: Values of HDL are in mg/dl and represented in mean ± SD.
(a=p<0.05 vs Atenolol + GM treatment, b=p<0.05 vs GM treatment, c=p<0.05 vs Atenolol treatment).
Note: Values of VLDL are in mg/dl and represented in mean ± SD.
(a=p<0.05 vs Atenolol + GM treatment, b=p<0.05 vs GM treatment, c=p<0.05 vs Atenolol treatment).
Note: Values of LDL are in mg/dl and represented in mean ± SD.
(a=p<0.05 vs Atenolol + GM treatment, b=p<0.05 vs GM treatment, c=p<0.05 vs Atenolol treatment).
The effect of atenolol and Glycine max on the AST, ALT and its ratio in serum sample is shown in Table 3. There was statistically significant difference in the level of AST, ALT and its ratio among the groups. Statistical analysis showed that there were significant alterations of atenolol induced rats compared to control rats. However, long term treatment with glycine max significantly decreased the level of AST and ALT compared to atenolol induced rats and insignificant difference to control rats.
Table 4 illustrate that long term exposure of atenolol induced the level of MDA and reduced the level of catalase and SOD in serum sample of experimental rats. Statistical analysis shows significant alterations in LPO, catalase and SOD in among groups. One-way ANOVA analysis demonstrated that long term treatment with glycine max showed significant decrease in the level of LPO and increase in the level of catalase and SOD compared with atenolol induced rats. However, treatment with glycine max shows antioxidant properties in experimental rats.
For decades and decades, people have relied on herbs and spices both for their culinary and medicinal purposes. There is mounting evidence from the scientific community that many of these herbs and spices do, in fact, have therapeutic characteristics that may improve symptoms or even prevent illness. Growing scientific evidence suggests that various culinary herbs and spices, including garlic, black cumin, cloves, cinnamon, thyme, bay leaves, rosemary etc. have antibacterial qualities that may be employed in medicine [45]. Hypertension may cause harm to the heart, blood vessels, kidneys, and other organs leads to various diseases, Medication and lifestyle changes may lower blood pressure. Atenolol a beta blocker used to treats hypertension with or without other medicines. It reduces angina and heart attacks by relaxing blood arteries and reducing heart rate, increases blood perfusion hence decreases blood pressure. Atenolol is still widely used despite the fact that it may cause hyperlipidaemia as its potential side effect [46]. The present research was planned to utilise atenolol in conjunction with glycine max to lower the burden of hyperlipidaemia and treat hypertension at the same time. This was done in an effort to fight the negative effects of this side effect.
Glycine max isoflavones has been reported to reduce heart disease risk by blocking LDL oxidation15 and enhances serum cholesterol clearance by activating SREBP-2. It also reduces insulin level, which affects hepatic SREBP-1 transcription factors that leads to the reduction of serum and hepatic LDL-C & VLDL-C level [47]. It is believed that the high phytosterol content of glycine max is responsible for its hypocholesterolemic action. This impact may increase cholesterol excretion by interfering with the biosynthesis of cholesterol [48]. Glycine max has a somewhat favourable inverse connection with Atenolol-induced blood TC, TG, LDL, and VLDL levels. Atenolol 10mg/kg elevated total cholesterol, triglycerides, LDL, and VLDL while lowering HDL. Atenolol in combination with Glycine max altered the drug-induced lipid profile. Animals given Glycine max and Atenolol showed reduced total, triglyceride, and LDL cholesterol levels and higher HDL cholesterol. Glycine max restores cellular components owing to high antioxidant and catalase levels, which protect tissues from peroxide assault. SOD defends against ROS. Glycine max treatment boosted SOD, catalase, and lowered MDA levels in atenolol-induced hyperlipidemic rats. Glycine max. restores the liver’s amino acid-protein metabolism. Long-term glycine max treatment in atenolol-induced hyperlipidemic rats decreased serum AST and ALT, indicating liver renovation. These findings demonstrated that Glycine max has hypolipidemic potential and had the ability to counteract the eleteriouss effects of Atenolol-induced hyperlipidemia. The findings of the current study indicated that the intake of Glycine max may reduce the risk of Atenolol-induced hyperlipidemia by as much as 50%.
In conclusion, soybean consumption, when compared to a control diet, substantially lowers both SBP and DBP and reduces hyperlipidemia induced by atenolol. The findings demonstrate that a combination of the beta-blocker medicine Atenolol with the amino acid glycine max is more effective in the treatment of both hypertension and reducing the deleterious effect of the drug atenolol. The glycine max can also be recommended to be used as prophylactic drug for the control of hyperlipidemia in normal and the patients taking beta-blockers.
This research work can be the foundation for further investigation in which the active constituents of glycine max responsible for decreasing hyperlipidemia can be isolated and can be prescribed along with beta blockers to combat drug induced hyperlipidemia.
The authors gratefully applaud to Director, Khalsa College of Pharmacy Amritsar for providing the essential facilities for conducting this research.


