Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Rafiga Mazahir Baghirova*
Received: April 17, 2023; Published: April 26, 2023
*Corresponding author: Rafiga Mazahir Baghirova, Department of “Medical and Biological Sciences”, Azerbaijan State Academy of Physical Education and Sport, Fatali Khan Khoyski Street 98, Baku 1072, Azerbaijan Republic
DOI: 10.26717/BJSTR.2023.50.007887
In order to study the participation of the medial nucleus of the septum in the implementation of the developed drinking habit, electrical and chemical stimulation (carbocholine, serotonin, norepinephrine) of the medial nucleus of the septum against the background of implementation drinking conditioned reflex skill. The data obtained indicate a certain role of the medial nucleus of the septum in the implementation of the drinking conditioned reflex habit.
Keywords: Medial Nucleus of the Septum; Electrical and Hemostimulation; Drinking Conditioned Reflex Skill
The septal region belongs to those structures in the limbic system that occupy an intermediate position between the phylogenetically old parts of the forebrain and the diencephalon and play an important role in the mechanisms of formation of emotional and motivational behavioral responses. The septal region plays an important role in eating and drinking behavior It has been shown, in particular, that destruction of the dorsolateral sections of the septum causes hyperphagia, while damage to its ventral regions causes aphagia with subsequent death of the animals [1-4]. The complex mechanism of participation of the spinal region in the organization of motivational eating behavior has not yet been sufficiently studied. The nuclei of the lateral, medial, caudal, and ventral groups are involved, including through connections with the hypothalamus, in eating and sexual behavior, the formation of dominant-subordinate relationships, and parental attachment to children [5-7]. With regard to drinking behavior, opposite effects are observed: irritation causes a decrease in water drinking, damage causes polydipsia. Apparently, the septal region also determines the nuances of appetite. A large number of experimental data points to the role of the nuclei of the septum in sexual motivation [8-9]. Based on the foregoing, in this series, the influence of electro-, chemostimulation, as well as temporary switching off of the medial nucleus of the septum on the execution of the drinking conditioned reflex skill was studied.
The studies were carried out on 25 Chinchilla rabbits weighing 2-3 kg, trained in the drinking conditioned reflex.
The introduction of neurochemical preparations was carried out in a volume of 0.005 ml of saline using a special device consisting of a micromanipulator and a syringe connected to an injection needle with a polyethylene tube, which makes it possible to inject chemical solutions into various brain structures under conditions of free behavior of the animal. The application was made in doses: carbocholine (CH) from 0.5 to 3 mcg; serotonin (5-OT) from 10 to 100 mcg, noradrenalin (NA) from 10 to 50 mcg. Temporary shutdown of the studied areas of the CNS was carried out with a 10% novocaine solution. To control the rabbits, a physiological solution was injected into the study area in a volume equal to the injected solutions.
Figure 1 Influence of electrical and chemical stimulation of the medial septal nucleus on the time parameters of the performance of the conditioned reflex drinking habit. 1 - background; 2.3 - low- and high-frequency stimulation; 4.5 - application of CH (small and large doses); 6.7 - introduction of 5-OT (small and large doses); 8.9 - NA application (small and large doses); 10 - the introduction of novocaine.
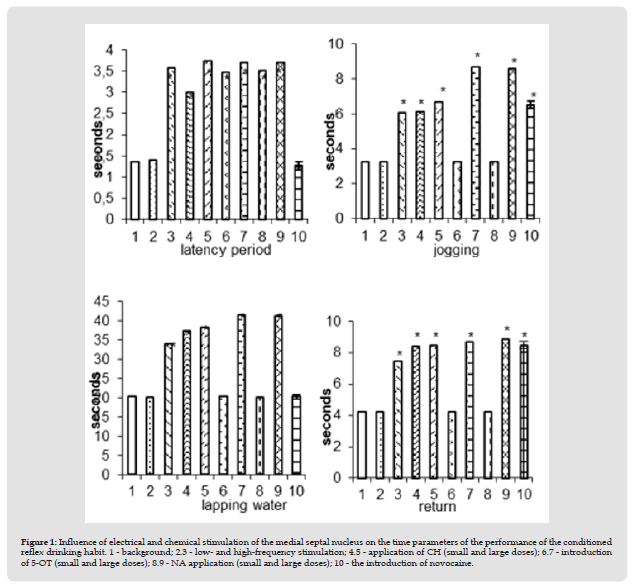
Execution of a conditioned reflex skill under conditions of electrical stimulation of the medial nucleus of the septum at low parameters of the irritating current (5 Hz; 50 μA; 0.5 ms) did not undergo significant changes. With an increase in stimulation parameters (10-20 Hz; 100 μA; 0.5 ms), some inhibition in the behavior of animals was noted, but in general execution skill was preserved. In this regard, the time spent on the latent period of the reaction to the conditioned stimulus slightly increased (from 1.27 ± 0.07 to 3.57 ± 0.1 sec), a run jump (from 3.27±0.07 to 6.07±0.1 sec), lapping (from20.27±0.11 to 34.1±0.18 sec) and reverse return (from 4.1±0.1 to 7.47±0.09 sec). The amount of water drunk did not change and remained at the level of background indicators (Figure 1-3). Changes in the behavior of animals were also noted with the use of neurochemical drugs. Thus, the application of CH to the medial nucleus of the septum also leads to some inhibition in the performance of the conditioned reflex drinking habit, as was noted in the case of electrical stimulation of this area.
The animal reacts to a suppressed sound signal with a longer latent period (3.0±0.07 instead of 1.2±0.07). Having jumped over the barrier, it does not tend to the drinker, despite the existing water deprivation, as a result of which the time of the run-jump increases from 3.23±0.07 to 6.13±0.09 sec. The time spent on lapping also increased (from 20.2±0.12 to 37.43±0.1sec) and return (from 4.07±0.1 to 8.43±0.11). The amount of water consumed does not change and remains at the background level. Full restoration of the conditioned reflex drinking habit was observed after 90-120 minutes. Increasing the dose of CK to 3 μg led to stupor, freezing, and alertness. The application of an increased concentration of CH led to even greater lethargy, stupor. All time parameters of the performance of the conditioned reflex drinking habit increased even more: the latent period - from 1.23±0.07 sec to 3.73±0.11 sec; jogging jump - from 3.3±0.07 to 6.67±0.08 sec; lapping - from 20.3±0.07 to 38.4±0.31 sec and reverse return - from 4.13±0.09 to 8.5±0.11 sec (Figure 1-5). The introduction of small doses.
The application of an increased concentration of CH led to even greater lethargy, stupor. All time parameters of the performance of the conditioned reflex drinking habit increased even more: the latent period - from 1.23±0.07 sec to 3.73±0.11 sec; jogging jump - from 3.3±0.07 to 6.67±0.08 sec; lapping - from 20.3±0.07 to 38.4±0.31 sec and reverse return - from 4.13±0.09 to 8.5±0.11 sec (Figure 1-5). The introduction of small doses of 5-OT (10-30 mcg) did not disturb the implementation of the developed drinking habit. When applied at a dose of 5-30 mcg, a reaction of alertness, passive fear is observed. Generally the execution of the skill persisted, there was an increase in the time of the latent period of reaction to the conditioned stimulus (from 1.27 ± 0.07 to 3.47 ± 0.09 sec). Increasing the dose of 5-OT to 50–100 μg led to inhibition in the behavior of animals, which was reflected in an increase in all time parameters. implementation of conditioned reflex drinking habit: latent period from 1.27±0.07 to 3.7±0.07 sec; jogging from 3.27±0.07 to 8.67±0.06 sec; lapping from 20.23±0.12 to 41.47±0.1 and reverse return from 4.3±0.07 to 8.7±0.07 sec (1-6.7).
Application of NA at a dose of 10-20 μg caused the appearance of a defensive reaction, passive fear, which led to an increase in the time of the latent period of reaction to a sound signal (from 1.27±0.07 to 3.5±0.1 sec). An increase in the dose of NA to 50 μg resulted in the animal freezing, which led to an increase in all time parameters for the execution of the conditioned reflex: the latent period, from 1.27±0.07 to 3.7±0.07 sec; jogging run - 3.23±0.07 to 8.57±0.1 sec; lapping - from 20.23±0.12 to 41.33±0.14 sec and reverse return 8.9±0.11 instead of 4.27±0.07 sec (Figure 1-8.9).The introduction of novocaine into the medial nucleus of the septum as a whole did not cause a violation of the performance of the developed skill. In comparison with intact rabbits, an increase in the orientingexploratory reaction of the animal was observed from the first minutes - the rabbit began to sniff, move around the chamber, rise on its hind legs, and sniff the starting compartment of the chamber. However, despite such an increase in motor activity, when a sound signal was given, the animal jumped and ran from the starting compartment of the chamber to the target one, followed by lapping. At the same time, the latent period of the appearance of the conditioned reflex remained unchanged (1.23±0.07 sec). In connection with the strengthening of the orienting-exploratory reaction of the animal, the time spent on jumping and running increased from 3.23±0.07 sec to 6.5±0.08sec. The lapping time remained unchanged and amounted to 20.23±0.12 sec. In connection with the emergence of an exploratory reaction -“examination” and sniffing of the target compartment of the experimental chamber, the time for the animal to return to the starting box of the chamber increased slightly from 4.23 ± 0.07 to8.5 ± 0.1 sec (Figure 1-10).
Novocaine blockade of the medial nucleus of the septum did not affect the amount of water intake. In the literature, there are numerous data indicating an increase in emotionality during the destruction of the septum. However, according to some scientists, these changes may depend on concomitant damage to the amygdala and the terminal strip since with the «pure» destruction of the above structures, an increase in emotionality is not observed [10]. In studies of some scientists, a reduction in the duration of paradoxical sleep with the destruction of the septum, violations of maternal behavior [11]. The same authors noted an increase in exploratory activity during the destruction of the septum,which coincides with our data. The appearance of a state of inhibition in the performance of the conditioned reflex drinking habit in the case of electrical stimulation of the medial septum and the intensification of the orienting- exploratory reaction, observed when this nucleus is temporarily turned off, apparently indicates the direct participation of the ulnar nucleus in the organization and formation of orienting-exploratory reactions. It should be noted that the appearance of a similar state of inhibition of the drinking habit was also observed by us in the case of the application of small doses of CK (0.5–2 μg) to the medial nucleus of the septum, and the occurrence of pronounced freezing and stupor when the dose was increased to 3 μg. Administration of acetylcholine to rats in the region of the septum blocked the conditioned fear response. According to the author, these violations are characteristic of conditioned reflex activity, since the unconditioned reaction is manifested in full. Blocking with atropine (10–20 μg) of the cholinergic structures of the septal region disrupts the development of a conditioned reaction of passive avoidance, but does not affect the development of a food reaction of alternating the choice of feeders and a conditioned active avoidance reaction; a slowdown in the extinction of the defensive reaction was noted. The effects of atropine are similar to the behavioral disturbance caused by a section of the septum. Since choline-sensitive areas are widely distributed within the limbic and other subcortical structures, behavioral changes in systemic cholinergic blockade are due to the influence of anticholinergic drugs on many areas of the limbic and other subcortical structures, among which, perhaps, the cholinergic structures of the septum play a dominant role.
In our experiments, the application of CH to the medial nucleus of the septum did not affect the amount of water consumed. An increase in drinking intake was noted by some scientists with the introduction of CH crystals into the lateral nucleus of the septum. However, some researchers argue that the effect depends on the penetration of CH into the ventricles and its action on the subfornical organ, others generally found a complete insensitivity of the «drinking system» to the introduction of CH, angiotensin II or histamine into any structures of the large limbic circle [9]. The application of small doses of 5-OT (5-30 μg) led to a reaction of alertness and passive fear. Increasing the dose to 50-100 mcg was accompanied by a state of inhibition. Inhibition of conditioned reflex activity was noted by some scientists with the introduction of 5-OT. An increase in the time of the latent period of the alimentary conditioned reflex was also noted with the introduction of the precursor of serotonin, 5- hydroxytryptophan. Moreover, the severity of inhibition and the duration of the effect depended on the dose of the substance [12]. The introduction of small doses of NA into the medial nucleus of the septum led to the emergence of a defensive reaction, a state of passive fear. Increasing the dose of NA led to the freezing of the animal. A similar inhibition of only food conditioned reflexes was noted by some researchers under the influence of NA. The occurrence of a state of stupor was noted during the application of adrenaline and norepinephrine into the ventricles of the cat’s brain. At the same time, in the experiments of some scientists with the introduction of adrenaline and NA into the septal region, it was shown that the drowsy state of cats was replaced by wakefulness, and a reaction of alertness arose [13]. The results presented in this section show a certain role of the medial nucleus of the septum in the implementation of the developed drinking habit.


