Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Girum Tefera Belachew*
Received: April 02, 2023; Published: April 24, 2023
*Corresponding author: Girum Tefera Belachew, Department of Biotechnology, College of Natural and Computational Sciences, Debre Birhan University, P.O. Box 445, Debre Birhan, Ethiopia
DOI: 10.26717/BJSTR.2023.49.007880
Bispecific antibodies (BsAbs) have been developed, with one antigen-binding site coordinated against the CD3 receptor (which activates cytotoxic T lymphocytes) and the other against specific antigens found on cancer cells (CD19, CD20, CD33, CD123, HER2, epithelial cell adhesion molecule [EpCAM], BCMA, CEA, and others). Bispecific antibodies (BsAbs) are unique immunological constructs that frequently recognize two distinct target antigens from multiple proteins. In the 1980s, the first advanced BsAb archetypes were developed. Using compound formation methods or quadroma innovation, comprising somatic hybridization of two antibody-secreting hybridoma cells, the first BsAbs were produced. In any event, the immunogenicity or complexity of their manufacture placed limitations on these approaches. Concentrating on two or more approved pathways can increase restorative viability and address the attractive and regular use of BsAbs in IBD. Furthermore, the extraordinary ability of BsAbs to act as bridging molecules between various therapeutic targets offers new ways to maximize their use. Soundly planned BsAbs employ a variety of therapeutic strategies, including the direct targeting of particular immune cell subsets, the colocalization and cross-connection of cell surface receptors to activate novel signaling, or the rerouting of the immune system to attack pathogenic cell subsets. Two BsAbs have so far been approved for usage in the US (blinatumomab; Amgen) and Europe (catumaxomab; Trion Pharma). It became possible to obtain many iterations of potential Ab derivatives for use in treatment thanks to the development of novel procedures for BsAb aging. This article aims to provide an overview of the therapeutic benefits of bispecific antibodies.
Keywords: Bispecific Antibody; Antibody Constructs; Cancer; Formats; Diagnosis
Bispecific antibodies (BsAbs) have been developed, with one antigen-binding site coordinated against the CD3 receptor (which activates cytotoxic T lymphocytes) and the other against specific antigens found on cancer cells (CD19, CD20, CD33, CD123, HER2, epithelial cell adhesion molecule [EpCAM], BCMA, CEA, and others) [1]. BsAb binding activates cytotoxic T cells and speeds up the destruction of cancer cells by bringing together cytotoxic T lymphocytes and cancer cells. In addition to broad-reaching BsAbs directed against cancer cells, numerous bispecific compounds have been developed for the treatment of various diseases. The Wnt signal-transduction pathway’s components (sclerostin and Dkk1) are inhibited by the BsAb used to treat osteoporosis, which also enhances osteoblast differentiation and bone tissue growth [2]. Blood-coagulation factors IX and X are linked by ACE910, which is meant to reduce hemophilia A patients’ bleeding rates. The coagulation cascade is enhanced by the assembly of coagulation factors [3]. A candidate for an antimedicine Alzheimer’s is a BsAb against the transferrin receptor, which allows entry through the blood-brain barrier, and protease BACE1, which aggregates amyloid peptides [4]. Autoimmune-related BsAbs typically link cytokines such TNF, IL1, IL4, IL14, IL17, IL23, and others [1,5]. It has been discovered that using two mAbs against cytokines at once has substantial side effects without primarily improving productivity in autoimmune diseases. In this way, BsAbs for the treatment of autoimmune illnesses usually combine two anti-cytokine antigen-binding sites and have a greater therapeutic potential than combining two mAbs [6,7]. The following cytokines, in particular, are most important for treating psoriasis: IL17, IL23, IL6, and TNF [8]. Clinical effects of ABT122 against TNF and IL17A on psoriatic joint inflammation and rheumatoid joint discomfort have been reported [9].
In contrast, COVA322’s Phase I/II clinical studies for treating psoriasis [10] (same specificity as ABT122) were terminated due to safety concerns [8]. The provocative cytokines IL1 and IL1, which are present in the cartilage and synovial fluid of osteoarthritis patients, are targeted by the antigen-binding sites of ABT981 [11] Over singlepurpose Abs, BsAbs have a number of significant advantages. BsAbs focus certain immune system effectors on cancer cells, increasing their cytotoxicity. As BsAbs interact with two different surface antigens in contrast to monospecific Abs, they can provide better binding specificity. It is possible to increase expenses by lowering the cost of development and clinical trials by using BsAbs instead of a combination treatment with two monospecific medicines. Since coexpression of multiple receptors has been discovered in many cancer cells and one disease modulator may play a crucial role in numerous independent pathways, targeting two different growth-promoting receptors on a single cancer cell may increase the antiproliferative effect and prevent the development of resistance [12,13]. More than 50 mAbs have been brought to the pharmaceutical market, and their annual sales have reached more than US$60 billion. According to a 2014 analysis, the market for corrective BsAbs will increase to $5.8 billion annually by 2024 [14]. The treatment of «fluid» malignancies, such as blood cancer, is coordinated with reparative BsAbs that have been approved for clinical use (leukemia and lymphoma). The advantage of «fluid tumors» is that, unlike solid tumors, they do not develop in the body and can therefore not be accurately studied (breast, uterus, rectum). Due to the fact that malignant leukocytes proliferate in the bone marrow and enter the blood circulation system as individual cells, rendering them susceptible to BsAb treatment, leukemia and lymphoma never produce the side effects typical of other oncological illnesses. BsAbs are being developed for a variety of diseases, including autoimmune (diabetes, asthma, and arthritis), infectious (pneumonia), hemophilia, and Alzheimer’s disease [6]. This article aims to provide an overview of the therapeutic benefits of bispecific antibodies.
What is a Bispecific Antibody (BsAbs)?
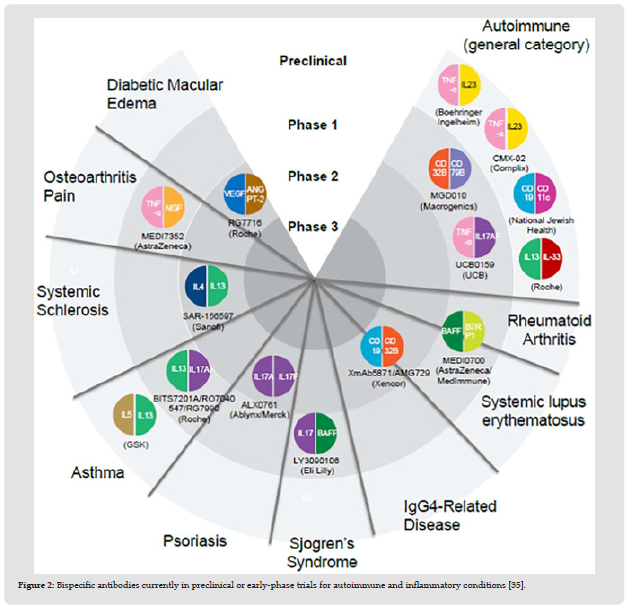
Bispecific antibodies (BsAbs) are exceptional types of immunizers that frequently recognize two different target antigens from multiple proteins [15,16]. In the 1980s, the first advanced BsAb archetypes were developed. Initial BsAbs were produced by somatic hybridization of two antibody-secreting hybridoma cells or by quadroma innovation procedures [15,16]. In any case, the immunogenicity or complexity of their manufacture limited these approaches [17,18]. BsAb development has recently had a comeback, which has been somewhat aided by innovative advancements in BsAb platforms supported by new molecular, structural, and computational biology approaches. All things considered, there are currently a large number of novel configurations (roughly 100) that are available (Figure 1); reviewed in [16,17,19-21], and more than 50 different BsAbs are currently in various stages of clinical development across a range of therapeutic signs, including oncology, autoimmune / inflammatory diseases (Figure 2), infectious diseases, and cardiovascular disease. BsAbs can be broadly divided into three major structural classes: antibody fragments lacking an Fc domain (such as diabodies and tandem single-chain variable fragments [scFvs]) [15,22-24]; alternative scaffold proteins fused to antibodies, antibody Fc regions, or human serum albumin to improve their pharmacokinetic (PK) properties or possibly valency (such as dual variabledomain immunoglobulins-G [DVD-Igs]); and antibody fragments [16,21,25-32]. Each of these formats has unique advantages of its own. Tetravalency, for instance, is produced by the Fc combination BsAbs and may be important for power and crosslinking [33]. Fully IgG BsAbs are frequently capable of attracting antigens with a single arm, which has advantages that will be discussed in greater depth later. Fully IgG BsAbs also mimic IgG4 BsAbs that develop spontaneously in people as a result of «Fab-arm exchange,» giving them biological precedence [21,34,35]. The development of numerous tailored BsAbs for the treatment of numerous autoimmune and inflammatory disorders (Figure 2).
Figure 2 Bispecific antibodies currently in preclinical or early-phase trials for autoimmune and inflammatory conditions [35].
Bispecific Antibodies: How Would they Work?
Concentrating on two or more approved pathways can increase restorative viability and address the attractive and regular use of BsAbs in IBD. Furthermore, the extraordinary ability of BsAbs to act as bridging molecules between various therapeutic targets offers new ways to maximize their use. Soundly planned BsAbs employ a variety of therapeutic strategies, such as the selective targeting of particular immune cell types, colocalization and cross-connection of cell surface receptors to trigger novel signaling, or immune system reprogramming to target pathogenic cell types[15,36].
Therapeutic Bispecific Antibodies
Two BsAbs have so far been approved for usage in the US (blinatumomab; Amgen) and Europe (catumaxomab; Trion Pharma). More than 60 drugs are undergoing preclinical testing, and 30 are currently through clinical testing, with 2/3 of them being used to treat malignant growth [37]. T lymphocytes are brought closer to cells communicating specific antigens on the surface by two restorative BsAbs that are readily available, and by a select few drugs in clinical and preclinical research, which also bind two antigens on the surface of a target cell.
Therapy with BsAbs made from all-encompassing IgG molecules displays active immunity against specific cancer cells, which can later lead to broader use of this format to provide enduring anticancer immunity in the body. Due to catumaxomab, dendritic cells that introduce the antigen have a preference for the fragment crystallizable position, which causes a protracted immune response to begin following therapy [38]. Vaccination occurs as a result of the setting up of an immuno-activating milieu that attracts and initiates dendritic cells This is because BsAbs produced by synthetic heteroconjugation (where the Fc of two IgG molecules are covalently linked) are responsible for this [39].
Bispecific Antibodies Blocking Signaling Pathways
Receptor Tyrosine Kinases (RTKs) are a category of cell-surface development factor receptors that are mostly linked to oncogenesis. Several monospecific RTK-targeting antibodies, including cetuximab (for colorectal cancer) and imatinib (for CML and GIST), as well as herceptin (for metastatic breast cancer), have been approved for the treatment of malignant tumors. Yet, a variety of signaling pathways with exceptional or concealing abilities are involved in pathogenesis. As a result, killing two targets simultaneously with one molecule has tremendous allure and offers a better therapy potential than mAbs [40].
HSA Body Bispecific Antibodies: The bsAb MM-111 combines two scFvs to produce an adjusted HSA [41]. It simultaneously focuses on the HER2/HER3 signaling pathways. HER2 is a recognized target for a number of cancerous growths. An important tool for pharmaceutical protection against HER2 inhibitors is HER3 flagging.
HER2/HER3 double concentrating may result in a more effective response. Patients with HER2-positive solid tumors are being tested in clinical studies for MM-111 either alone or in combination with trastuzumab [42].
ScFv-IgGs: Further bsAb MM-141 targets HER3 and the IGF-IR, as well as the insulin-like growth factor I receptor (IGF-IR). MM-141, in contrast to MM-111, is an IgG-like bsAb with two scFvs attached to the IgG’s consistent region. The PI3K/AKT/mTOR pivot, a mechanism for targeted resistance, is started by both IGF-IR and HER3. MM-141 interacts with HER3 and IGF-IR, impeding the resistance mechanism [43]. MM-141 is now being studied in stage I in hepatocellular cancer patients.
Two-in-one Antibody: A two-in-one (DAF) phage-determined and modified counter antibody is called duligotuzumab. It is connected to EGFR and HER3, which inhibits the HER-downstream family’s signaling pathways [44]. Human malignant cancers such head and neck and colorectal malignant tumors are caused by deregulated EGFR- and HER3- needy signaling. People who frequently receive the EGFR inhibitor cetuximab develop anti-EGFR resistance. When combined with radiation, duligotuzumab overcomes medication resistance and strengthens the effects of radiation. Duligotuzumab is now being tested in clinical trials for individuals with neck squamous cell carcinoma and epithelial tumors. In a stage II investigation involving patients with intermittent or metastatic neck squamous cell carcinoma, duligotuzumab demonstrated equivalent anti-cancer effect to cetuximab [45]. Patients receiving duligotuzumab should be aware that they are highly susceptible to side effects include febrile neutropenia, hypokalemia, nausea, and dehydration [46].
Angiogenesis is a critical cycle in the growth and spread of tumors. Cancer angiogenesis is influenced by a number of angiogenic factors, including endothelial development factor receptor2, VEGFR3, endothelial development factor A, angiopoietins, and plateletdetermined development factors. By emptying these proteins, many cancer treatments disrupt angiogenesis [47]. Results are predominant when angiogenic factors are focused twice [48]. A human IgG1like CrossMab called RG7221 targets VEGFA and angiopoietin-2, two essential angiogenic factors (Ang-2). RG7221 effectively inhibited angiogenesis and tumor growth in preclinical models, having a greater impact than single-pathway inhibitors [49]. RG7221 is now being studied in stage II patients with colorectal cancer. Patients receiving RG7221 medication may experience the negative effects of hypertension, asthenia, migraine, and tiredness, according to clinical data from the stage I research. In stage II testing in individuals with wet age-related macular degeneration (wet AMD), RG7716 is a comparable CrossMab that was also designed to prevent VEGFA and Ang2 [49].
Many cytokines have been identified as important diseasecausing agents in autoimmune and provocation disorders [50]. Hence, preventing these cytokines holds potential as a therapeutic. For example, blocking TNF- has important therapeutic benefits for conditions like psoriasis, psoriatic joint discomfort, Crohn’s disease, ulcerative colitis, teenage joint inflammation, and a wide range of infections [51]. The following are also recognized cytokines: IL-6, IL- 17, IL-1, IL12, TGF-, IL-4, and IL-13. [52-55].
Nanobodies
The Ablynx-developed ozoralizumab (ATN-103) is a tiny trivalent, bispecific nanobody with a strong affinity for both HSA and TNF-. The serum half-life of albumin is extended by conjugation [56]. After completing stage II clinical studies in rheumatoid joint inflammation patients, ozoralizumab showed a significant reduction in the condition. Ozoralizumab has unique molecular characteristics that make it appealing for clinical uses, including small size, low immunogenicity, and long serum half-life. Comparable nanobodies produced by Ablynx include ALX-0061 for rheumatoid arthritis joint inflammation and ALX-0761 for provocative sickness [57,58].
SAR156597
Tetravalent bispecific pair IgG SAR156597 binds to both IL-13 and IL-4 simultaneously. SAR156597 is an anti-IL4 IgG molecule with its N terminus tangled up in the variable region of an anti-IL13 antibody [59]. It has successfully completed the idiopathic pneumonic fibrosis stage I/II clinical assessment [60].
The transportation of payloads including drugs, radiolabels, and nanoparticles is an intriguing application of bsAbs. Once the unbound Bispecific particles have been removed from the blood circulation system, the payloads are controlled. In cancer locations, bispecific particles can be used to improve payloads [26,61-63]. The overall effect of this technique is to prolong serum maintenance and increase the cancer/blood ratio.
DNL
For radioimmunotherapy and cancer imaging, the DNL technique’s bispecific TF2 assembled is used. It specifically links to CEA and the histamine-succinyl-glycine hapten identified by 99mT (HSG). In the preclinical trial, TF2 was first infused, and after the elimination of the bsAb from the circulation, 99mT-marked HSG was directed. With high cancer take-up of 99mT, a high cancer/blood proportion was observed. TF2 research in patients with colorectal malignant tumors is currently in stage I [64]. Focusing against 177Lu HSG/111InHSG and CEA for radioimmunotherapy in patients with colorectal neoplasms and focusing against [65] are some more uses of TF2. Immuno-positron emanation tomography with GaHSG and CEA.
Immunogenicity and Fixed Stoichiometry
The development of adapted (90% human) and fully human (100%) antibodies has advanced protein design, and these antibodies are frequently associated with lower immunogenicity than chimeric (70% human) antibodies, such as infliximab [63,64]. While nonlocal and fragmentbased arrangements may increase the risk of immunogenicity in comparison to more local arrangements like IgG architecture, all protein medicines may encourage the development of anti-drug antibodies, especially with increased use [65,66]. Despite their earlier openness to biologics, patients with autoimmune and inflammatory diseases have been found to produce autoantibodies that could react with therapeutic antibodies [67]. Large immune structures that result from bispecific target commitment could also trigger «warning signals» that activate immunogenicity. But, continued clinical and preclinical experience should provide more clarity. Another potential barrier to BsAbs is their fixed stoichiometry, which prevents adaptive dose change of each component antibody in accordance with patient needs [20,67]. Because of this, dosing considerations for BsAbs may differ from those for conventional antibodies, with the primary objective being assurance of a safe and effective dose that may feat the additional substance or potential synergistic impacts of focusing on double pathways, as opposed to the ideal focusing of individual pathways. To coordinate dosing regimens for each target, however, imaginative designing of the BsAb’s affinity and avidity towards each of its aims may be helpful.
Regulatory and Technical Challenges
The development of mAbs over the past 20 years has greatly increased our understanding of the operational requirements for antibody-based healing [68]. From an administrative standpoint, the development of BsAbs is, in terms of characterization, quality, efficacy, and safety, fundamentally equivalent to that of any mAb [69,70]. But, because of their unpredictability and heterogeneity, they also bring a unique set of challenges. Preclinical toxicity studies, for instance, can be problematic given that the two targets ought to be present in the animal model [68,69]. Large-scale production Good Manufacturing Practice conditions and strength may both be problems for some bispecific structures [71,72]. Although there are challenges, good manufacturing practices are being strengthened in BsAb production [73]. Compared to typical mAb therapies, bispecific antibodies have innately more complicated systems. These systems include the possibility for larger and also more complex immune complexes, the beginning of cell-cell interactions, and the fusion of the liabilities of the two targets. Thus, it is anticipated that administrative specialists will thoroughly evaluate a molecule’s overall viability and security at various dosages, with strong justification required to assist with dose selection. Despite these challenges, the development of BsAbs in oncology has benefited from administrative offices acknowledging that bispecific moieties are not discrete substances, unlike blend drugs, which require demonstration of efficacy and safety for each individual component before their demonstration in combination [74,75].
Despite the fact that all patients with inflammatory bowel disease suffer discomfort, each patient’s experiences, disease profile, and response to treatment are very different [76]. In order to achieve greater restorative sufficiency than currently available biologics for the treatment of IBD, bispecific antibodies address the emerging field of healing antibodies and allow practitioners the opportunity to use contemporary focusing on techniques. The following strategies could be used to achieve this: enhanced or simultaneous blocking of target cytokines or pathways; specific targeting of specific cell types; novel signaling via receptor colocalization or hyper-crosslinking; or destruction of harmful cell types via T-cell redirection. BsAbs are thought to provide new options for individualized treatment in IBD, taking into account a patient’s own distinctive immunological profile. Although the use of BsAbs in the treatment of autoimmune and inflammatory illnesses is still in its infancy, recent advancements in their design have made it possible to upgrade molecules that are stronger and, in some cases, more IgG-like. Two BsAbs are already commercially available, and there is a diverse and extensive pipeline of more than 50 compounds in various stages of clinical development [77] While difficulties relating to cost have yet to be clarified, a single therapeutic agent that can be produced by two mAbs may have advantages from this perspective [74]. BsAbs offer promising therapeutic applications and are anticipated to prominently feature in the future for the treatment of IBD, despite the fact that there are still many challenges.
It became possible to obtain many iterations of potential Ab derivatives for use in treatment thanks to the development of novel procedures for BsAb aging. The following BsAbs differ from regular IgG in terms of pharmacokinetics, blood serum half-life, ability to infiltrate tumors, size, valence, and Fc content. BsAbs can have a synergistic effect that would not be possible with a combination of monospecific Abs due to the simultaneous obstruction of numerous biological pathways. The results of recent years show that novel BsAbs targeted against a variety of diseases, in which concurrent binding of numerous specific antigens can assume a vital role, will be developed in the near future by combining previously developed techniques. The «next generation» of diagnostic tools may benefit from the use of BsAbs. BsAbs are an important subject of additional research in biomedicine, pharmacology, and diagnostics due to their capacity for the simultaneous discovery of multiple antigens or the merging of antigen-binding sites with test markers.
All of the required data will be available upon request to the corresponding author.
The author wrote the review article alone.
The author is grateful to thank those individuals who gave help directly or indirectly.
There is no financial support and sponsorship.
There are no conflicts of interest.


