Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Hagar M Zayed1,2, Ola M Ezzatt1,2*, Nevine H Kheir El Din1, Ashraf M Abu Seida3 and Asmaa A Abo Zeid4
Received: April 01, 2023; Published: April 24, 2023
*Corresponding author: Ola Mohammed Ezzatt, Department of Oral Medicine, Periodontology and Oral Diagnosis, Faculty of Dentistry, Ain-Shams University, 20 Organization of African Union St., Cairo, 1156, Egypt
DOI: 10.26717/BJSTR.2023.49.007876
Radiation used in the treatment of head and neck cancer causes significant damage to the salivary glands and a loss of salivary function. Current therapies for radiation-induced salivary hypofunction aimed to stimulate residual functional salivary gland tissues and treat the symptoms of xerostomia without restoring the lost tissue. Several research strategies are emerging, aimed at regenerating salivary glands damaged by radiation via either gene therapy or by transplantation of stem cells. This concise review focuses on cell-based therapeutic strategies for regeneration of irradiated salivary gland (SG) tissues and discuss the possible regenerative potentials and applications of GMSCs in this context.
Keywords: Head and Neck Cancer; Gingival Mesenchymal Stem Cells; Radiotherapy; Salivary Glands; Transplantation; Xerostomia
Head and neck cancer is one of the most common types of cancer worldwide, the current standard of care includes surgical resection of the tumor followed by chemotherapy and radiation [1]. Radiation causes significant damage to the salivary glands and a loss of salivary function [2]. Consequently, irradiated patients’ quality of life decreases tremendously as a result of loss of saliva production, where they exhibit severe swallowing and speech disabilities, considerable pain and discomfort, mucosal infections, rampant formation of dental caries and severe accelerated periodontal disease resulting in loss of teeth [3].
The objectives of the current therapies are to protect the salivary gland during radiotherapy, stimulate the remaining salivary gland function and treat the symptoms of xerostomia. Current and emerging therapies for radiation induced xerostomia are summarized in (Table 1) [4]. These protocols of treatment involve the administration of artificial saliva substitutes or sialagogues to increase the flow rate of saliva [5]. In addition, parasympathomimetic drugs such as pilocarpine and cevimeline, have been used to stimulate residual functional salivary gland tissues [6]. Unfortunately, those therapies are short-lived and have multiple negative side effects of their own including nausea, diarrhea, and excessive sweating [7]. Therefore, novel therapeutic approaches to restore salivary gland function and the quality of life and to decrease the financial burden for these patients are required [8].
Tissue Engineering Approaches for Radiated Salivary Glands
Tissue engineering is a new area of science, its goal to unravel clinical and surgical problems associated with tissue loss and organ failure, by replacing or regenerating human cells, tissue or organs, to establish normal function [9]. Salivary gland regeneration has the potential to permanently restore salivary gland secretory function in patients with hyposalivation to improve their oral health and quality of life. The three main approaches that have been proposed are: Gene therapy, by using viral vectors, stem/progenitor cell-based therapy and replacement with a bioengineered gland [10].
Stem Cell Approach for Regeneration of Radiated Salivary Glands
The clinical success of salivary gland regeneration using cellbased approach requires support of the stroma, nerves, vasculature, and immune system. For cell transplantation to be feasible, the transplanted cells have to attach and survive in the damaged region. Furthermore, transplanted cells should be integrated into the salivary gland and be able to differentiate into a salivary gland cell lineage [11]. Stem cells have received great interest as potential therapeutics for a range of chronic, debilitating diseases that don’t have effective therapeutic options. A ‘‘stem cell’’ refers to a clonogenic, undifferentiated cell that’s capable of self-renewal and multi-lineage differentiation [12]. Interactions among these stem cells initiate and regulate developmental processes, resulting in the formation of highly specialized functional tissues and organs, once the organism matures, the pluripotent embryonic stem cells evanesce and some multipotent adult stem cells remain in the developed tissue to sustain the homeostasis and repair injuries [13]. Stem cells origins and organogenesis are demonstrated in (Figure 1). Different types of stem cells have been used as a regenerative treatment modality to ameliorate radiation-induced salivary hypofunction as summarized in (Table 2) [14-25]. Despite all promising results from stem cell therapies there were limitations with their use as with bone marrow derived stem cells (BM-MSCs), [26-31] the process of isolating BMMSCs from the bone marrow is expensive and invasive operation will be needed which is considered one of the great drawbacks of BMMSCs isolated from the iliac crest [32]. Furthermore, the expansion capacity seems to be restricted, since cells tend to age and lose their properties with repeated passaging and culture time in their multidifferentiation potential [33].
Figure 1 The embryonic stem cells undergo differentiation into multipotent stem cells. Interactions among these stem cells initiate and regulate developmental processes, resulting in the formation of highly specialized functional tissues and organs. Once the organism matures, the pluripotent embryonic stem cells evanesce and some multipotent adult stem cells remain in the developed tissue to sustain the homeostasis and repair injuries.
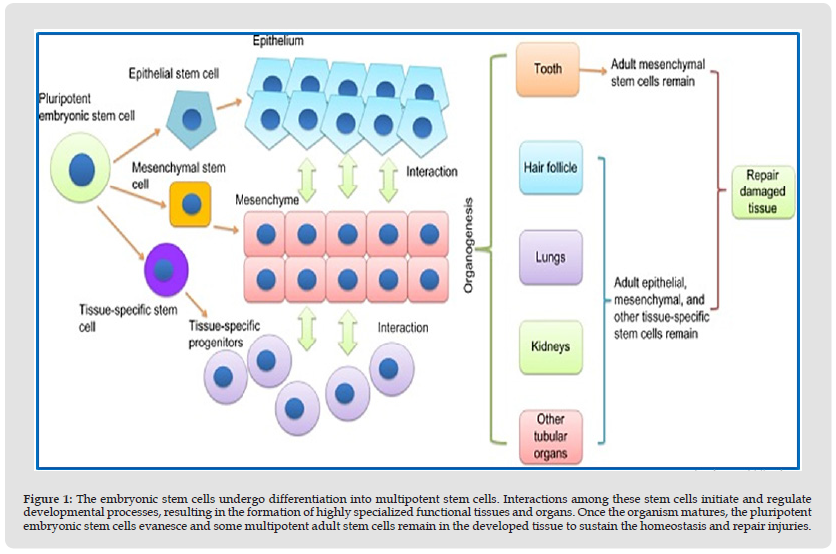
Table 2: A review of researches demonstrated regenerative potential of different types of stem cells in managing radiation-induced salivary hyposalivation.
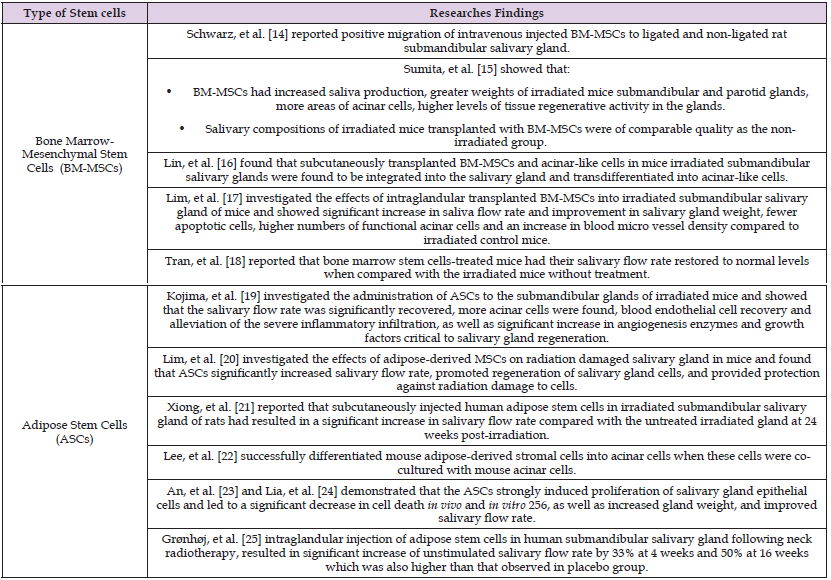
Oral derived tissues as sources of stem cell therapy for radiated salivary glands have also been investigated as documented in (Table 2). But major limitation for salivary gland regeneration in treating radiation-induced hyposalivation is the difficulty in isolating autologous stem cells from a severely injured gland [34]. Restriction in handling salivary gland stem cells due to their relatively short life span during in vitro cultivation resulted in narrowing the time window for implantation [35].
Gingival Mesenchymal Stem Cells for Salivary Glands Regeneration
Gingival mesenchymal stem cells (GMSCs) exhibit clonogenicity, self-renewal, and multipotent differentiation capacity, and these cells possess both stem cell-like and immuno-modulatory properties as compared to BM-MSCs, GMSCs show a faster proliferation rate [36]. Unlike BM-MSCs, which demonstrate abnormalities of cellular aging at 8–10 passages, GMSCs retain a stable morphology, maintain normal karyotype, do not lose MSCs’ characteristics at higher passages, and are not tumorigenic. Besides the well-established self-renewal, multipotent differentiation, and tissue regeneration capabilities, GMSCs possess outstanding immunomodulatory properties, which could be of great therapeutic interest. This allows GMSCs to ameliorate inflammatory diseases therapeutically, through their influence on the local microenvironment [37]. Zhang, et al. [38] demonstrated the value of treating wounds with GMSCs through their systemic infusion for wound repair and reported that besides their local enrichment in multipotent and self-renewing at the wound site, one of the mechanisms by which GMSCs were assumed to improve repair is via their modulation of the local inflammatory response. The therapeutic effect of GMSCs was thought to be mediated through the suppression of inflammatory infiltrates and inflammatory cytokines/mediators, the increased infiltration of regulatory T-cells, and the expression of anti-inflammatory cytokine IL-10 at the injured sites [36].
Table 3: Researches demonstrated regenerative potential of oral tissues derived stem cells in managing radiation-induced salivary hyposalivation.
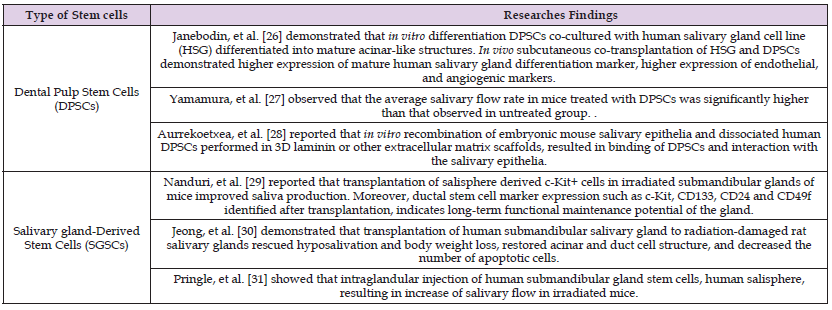
Gingival-derived mesenchymal stem cells (GMSCs) have shown remarkable tissue regenerative potential, noteworthy immunomodulatory properties and promising primary experimental therapeutic applications [39]. An interesting study was demonstrated by Abd El-Latif, et al. [40] who studied the regenerative potential of GMSCs in partially dissected rats’ submandibular salivary gland, they reported that GMSCs combined with fibrin glue at the surgically created defect site have resulted in restoration of the gland architecture showing deeply stained serous acini, reduction in granulation tissue, multiple blood vessels formed around well-defined ducts. Unfortunately, there is a lack of knowledge in the regenerative potential of GMSCs in treating salivary hypofunction secondary to irradiation (Table 3).
Research in the regenerative potential of GMSCs, as well as preclinical studies to investigate optimum concentration and suitable route for transplanted cells seem to be critical for clinical translation of this cell-based therapy for restoring the structure and function of irradiated salivary glands.
The authors want to acknowledge research team in Central Lab of Stem Cells and Biomaterial Applied Research (CLSBAR) in Faculty of Dentistry, Ain Shams University which is supported by Science, Technology & Innovation Funding Authority (STDF) under grant [CB project ID (21747)].
None declared.
The researchers declare that they did not receive any funding from public, commercial, or not-for-profit agencies.


