Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Takahiro Hirabayashi1, Ai Kimura1, Junko Shibato1, Michio Yamashita2, Fumiko Takenoya2* and Seiji Shioda3*
Received: April 05, 2023; Published: April 18, 2023
*Corresponding author: Seiji Shioda, Department of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Shonan University of Medical Sciences, 16-48, Kamishinano, Totsuka-ku, Yokohama, Kanagawa 244-0806, Japan
Fumiko Takenoya, Department of Sports Sciences, School of Pharmacy, Hoshi University, 2-4-41 Ebara, Shinagawa-ku, Tokyo, 142-8501, Japan
DOI: 10.26717/BJSTR.2023.49.007865
Currently, there are only a limited number of treatments approved to treat hair loss, and most of these are associated with a range of side effects. Research into novel therapeutic agents capable of promoting hair growth with fewer side effects is therefore necessary. Oxidative stress is a leading cause of hair loss due to its negative effects on scalp skin. Using antioxidants to reduce oxidative stress could therefore be potentially beneficial in promoting hair growth. As Indian head cactus (Parodia ottonis ssp. ottonis) has considerable antioxidant activity and contains substances such as adenosine that have been reported to be effective in promoting hair growth, we hypothesized that an extract of this plant might be effective in promoting hair growth. In this study, we evaluated the hair growth-promoting effects of cactus extract on human follicle dermal papilla cells (HFDPCs). Our results showed that cactus extract significantly increased cell proliferation and induced the mRNA expression of hair growth related genes including FGF-7 and VEGF. These results suggest that cactus extract may promote hair growth via the stimulation of HFDPCs and demonstrate the potential for use as a new hair growth promoting therapy.
Keywords: Cactus; Hair Growth; FGF7; VEGF; Dermal Papilla Cells
Abbreviations: FGF7: Fibroblast Growth Factor 7; GAPDH: Glyceraldehyde 3-Phosphate Dehydrogenase; HFDPCs: Human Follicle Dermal Papilla Cells; VEGF: Vascular Endothelial Growth Factor; ROS: Reactive Oxygen Species; FDA: Food and Drug Administration; DHT: Dihydrotestosterone; PCR: Polymerase Chain Reaction; RT: Reverse Transcription
Hair loss, or alopecia as it is known by in medical terminology, is a common condition that affects both men and women and is manifested by the loss of hair or absence of regrowth in some or all areas of the scalp. Although hair loss is generally not a life-threatening event, it can significantly impact on the mental health of affected individuals, rendering social interactions more difficult and causing psychological stress. Hair loss is caused by genetic factors and multiple external factors, including psycho-emotional stress, hormonal imbalance, lack of nutritional balance, intake of certain medications, and surgery [1,2]. Approved treatments for hair loss are nevertheless limited, perhaps in part due to this multiplicity of external factors potentially involved in the onset of this chronic condition. The development of new therapeutic agents to prevent hair loss and to promote hair growth has taken on considerable importance as a health and aesthetic issue throughout much of the world.
Oxidative stress is a major cause of hair loss and is likely to result from many sources including increased reactive oxygen species (ROS) production and declining endogenous antioxidant responses with aging. Hair follicles in the scalp become damaged by this chronic exposure to oxidative stress, with a reduced production and release of vital substances by the hair follicle cells resulting in ongoing hair loss [3]. Reducing oxidative stress in the scalp by employing antioxidants may therefore help to improve scalp conditions necessary for hair retention, and thereby prevent hair loss. To this end, previous studies have demonstrated that plant-derived antioxidants can reduce oxidative stress and could provide contributing factors to promote hair growth [4].
The Cactaceae plant family is diverse and contains many genera whose extracts are used in food, medicine, and cosmetics; many studies have reported a range biological activity displayed by compounds derived from cactus species [5]. For example, the Cactaceae plant family contains several antioxidants that protect lipid and protein oxidation from various oxidative damage, including polyphenols, flavonoids, ascorbic acid, carotenoids and betalains [6]. In our previous study, the extract of Parodia cactus, one of the major genera of the cacti family, was analyzed by capillary electrophoresistime- of-flight mass spectrometry (CE-TOFMS) and adenosine was identified as a substance associated with hair growth [7,8]. In addition, B vitamins such as pantothenic acid, pyridoxal, pyridoxamine, pyridoxine and riboflavin, which are known to be involved in hair growth, were detected [8,9]. Based on these results, we hypothesized that an extract of Parodia cactus could effectively promote hair growth and prevent hair loss. In this study, we evaluated the hair growth-promoting effects of extract produced from the Indian head cactus (Parodia ottonis ssp. ottonis) on follicle dermal papilla cells.
Cell dry powder was obtained from the whole Indian head cactus (Parodia ottonis ssp. ottonis), which was harvested by Gikoen Co., Ltd (Gifu, Japan) following the low-temperature vacuum extraction method [10]. One gram of cell dry powder was suspended in 10 ml of H2O and centrifuged at 1,500 rpm for 10 minutes. The resulting supernatant was purified by filtration through a 0.22 micrometer pore filter (Merck Millipore) and used as the cactus extract in these experiments.
Human follicle dermal papilla cells (HFDPCs) were purchased from Cell Applications, Inc. The cells were incubated in culture medium (TOYOBO Inc.) in type I collagen-coated dishes at 37°C under a 5% CO2 atmosphere.
HFDPCs (5 × 104 cells/well) were seeded in type I collagen-coated 96-well plates and cultured overnight. Cells were treated for three days in concentrations of cactus extract ranging from 0.001 to 10% v/v. Cell proliferation was examined using Cell Count Reagent SF, based on the WST-8 assay (Nacalai Tesque) according to the manufacturer’s instructions. The optical density value after the addition of reagent SF was measured using a Varioskan MicroPlate Reader (Thermo Fisher Scientific) at a wavelength of 450 nm. The mean optical density value of the non-treated control group was arbitrarily set to 100%.
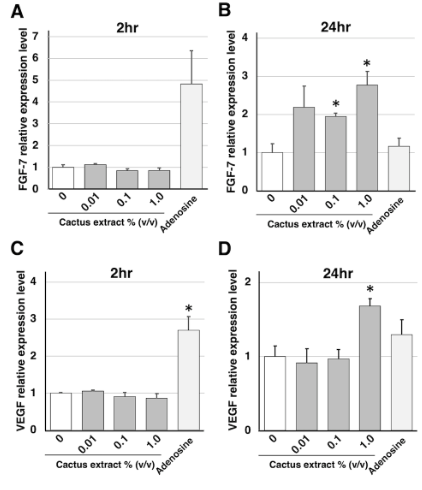
HFDPCs were seeded in type I collagen-coated 48-well plates (1.5×105cells/well) and incubated for 24h, then incubated for 2 and 24 h in medium containing different concentrations of cactus extract (0.001 to 1.0% v/v) and 2% adenosine as the positive control. Isolation of total RNA from HFDPCs and reverse transcription (RT) were performed using the FastLane Cell cDNA Kit (Qiagen). The polymerase chain reaction (PCR) primer set was as follows: human fibroblast growth factor 7 (FGF-7) primers, forward; 5’-TCT GTC GAA CAC AGT GGT ACC TGA G-3’, reverse; 5’-GCC ACT GTC CTG ATT TCC ATG A-3’, human Vascular endothelial growth factor (VEGF) primers, forward; 5’-AAA GCA TTT GTT TGT ACA AGA TCC G-3’, reverse; 5’- CTT GTC ACA TCT GCA AGT ACG TTC G-3’, and human Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers, forward; 5’-CAT CCC TGC CTC TAC TGG CGC TGC C-3’, reverse; 5’-CCA GGA TGC CCT TGA GGG GGC CCT C-3’. Quantitative real-time PCR was performed using SYBR Premix Ex Taq reagent (TaKaRa BIO Inc.) and a LightCycler 96 Instrument (Roche Molecular Systems, Inc.). Relative gene expression was calculated using the comparative delta Ct method with Ct values normalized to GAPDH as the housekeeping gene.
Results were expressed as the mean ± standard error of the mean (SEM). Differences in the data sets were analyzed by using the student’s t-test. P-values less than 0.05 (p<0.05) were considered to be statistically significant.
To investigate the effect of cactus extract on hair growth, we first determined its impact on the proliferative activity of HFDPCs. HFDPCs were incubated for 3 days in the presence of different concentrations of cactus extract (0.001 to 10% v/v), and cell proliferation activity was determined by WST-8 assay. Cell proliferation rates for each concentration are shown in (Figure 1) expressed as a percentage relative to control (untreated cells). Proliferation was found to be significantly increased compared to control even at the highest concentrations used, suggesting that the cactus extract was not cytotoxic to the HFDPCs. Cactus extract increases the expression of hair growth-related genes FGF-7, also known as keratinocyte growth factor (KGF), regulates epidermal proliferation via a paracrine mechanism to enhance hair growth. In addition, VEGF secreted from follicle dermal papilla cells promotes their proliferation via an autocrine mechanism [11,12]. The expression of these growth factors in HFDPCs was therefore examined by quantitative real-time PCR at 2 h (Figures 2A & 2C) and 24 h (Figures 2B & 2D) post-exposure of the cells to cactus extract. We found that FGF-7 mRNA levels were significantly increased in the HFDPCs 24 h after treatment with the extract at concentrations of 1.0% v/v (Figure 2B), while VEGF mRNA was also significantly increased 24 h after treatment with cactus extract at the 1.0% v/v concentration (Figure 2D).
Figure 1: Effects of Cactus Extract on Cell Proliferation of HFDPCs. Incubation of HFDPCs with Different Concentrations of Cactus Extract Enhanced the Proliferation of These Cultured cells as Determined by WST-8 assay. Values are means + SEM; n = 3 Experiments Per Concentration; *p < 0.05, **p < 0.01, vs. control.
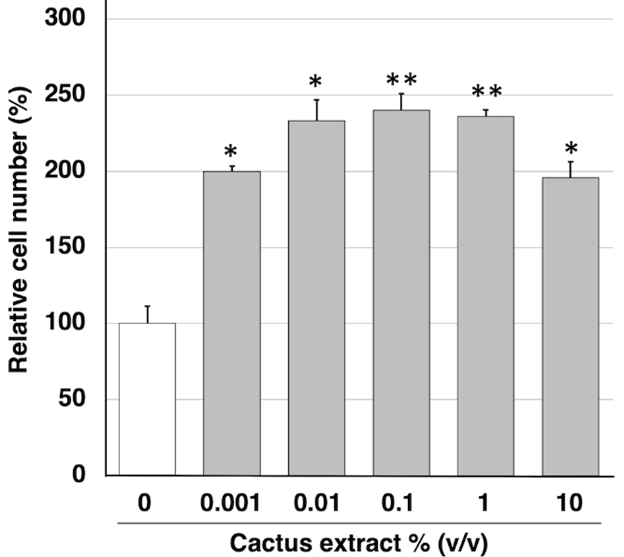
Figure 2: Effects of Cactus Extract on Expression of Hair Growth-Related Genes in Cultured HFDPCs. (A, B) FGF-7, (C, D) VEGF. HFDPCs were incubated for 2 h (A, C) and 24 h (B, D) in Medium Containing Different Concentrations of Cactus Extract. Adenosine was Used as the Positive Control. Values are means + SEM; n = 3 Experiments per Concentration; *p < 0.05, vs. Control.
Alopecia is a generally undesirable health problem in many societies around the world, with only a limited number of treatments available despite the increasing prevalence of hair loss. Oral finasteride and topical minoxidil, the only two agents approved by the Food and Drug Administration (FDA) for the prevention of hair loss and promotion of hair growth, are the most popular treatments in many countries for patients with alopecia [13]. Finasteride is a 5α-reductase inhibitor, which can reduce the conversion of testosterone to dihydrotestosterone (DHT), the principal androgen associated with hair loss [14]. Minoxidil, on the other hand, is a vasodilator that acts by opening potassium channels in the dermal papilla cell membrane, thereby enhancing hair growth by inhibiting apoptosis of these cells [15]. However, these treatments have several side effects. Finasteride has been associated with depression, selfharm, and erectile dysfunction. Minoxidil, on the other hand, has been associated with increased heart rate, difficulty breathing, rash and dermatitis‐like reactions [16]. Therefore, the development of novel therapeutic agents capable of preventing alopecia and enhancing hair growth with fewer side effects remains an unmet medical need. Given that Indian head cactus extract, with its high antioxidant capacity and hair growth components such as adenosine and B vitamins, was expected to have hair growth effects, we evaluated the hair growth promoting effects of cactus extract in HFDPCs in this study. Our results showed that cactus extract significantly increased cell proliferation by day 3 of treatment.
These results suggest that cactus extract promotes hair growth by stimulating HFDPCs and may therefore be beneficial in the treatment of hair loss. In addition, all concentrations of cactus extract, up to 10% v/v, increased cell proliferation. These results suggest that cactus extract is not toxic to HFDPCs. However, further human studies are needed to assess the safety and risks of cactus extract. Cactus extract also increased the expression of hair growth related genes FGF-7 and VEGF mRNA in this study. Cactus extract had no effect on FGF-7 mRNA expression at 2h but increased its expression more than adenosine at 24 h. This temporal difference in FGF-7 mRNA expression suggests that components other than adenosine in the cactus extract, such as antioxidants and B vitamins, are also involved. In addition to FGF-7 and VEGF, several other hair growth related genes have been reported, such as insulin-like growth factor 1 (IGF-1), hepatocyte growth factor (HGF) and TGF- [17-19]. To clarify the mechanism of hair growth effects by cactus extract, it is necessary to analyze the expression of these genes.
These results suggest that cactus extract may promote hair growth via the stimulation of HFDPCs and demonstrate the potential for use as a new hair growth promoting therapy. To our knowledge, the present study is the first to evaluate the effect of cactus extract on hair growth using cultured HFDPCs. These results are preliminary but important findings for the development of new therapeutics to treat hair loss. Further studies are needed in pre-clinical animal models and in early phase human to validate these findings in the clinical setting.
We would like to thank Sciencedit (www.sciencedit.com) for English language editing.
The authors declare no conflict of interest.
Conceptualization, T.H. and S.S.; Methodology, T.H. and S.S.; Validation, A.K. and J.S.; Formal Analysis, T.H., A.K. and J.S.; Investigation, T.H., A.K., J.S. and M.Y.; Resources, S.S.; Data Curation, T.H.; Writing - Original Draft Preparation, T.H.; Writing - Review & Editing; F.T. and S.S.; Visualization, T.H.; Supervision, S.S.; Project administration, S.S.; Funding acquisition, T.H. and S.S.
This research was in part supported by the Japan Society for the Promotion of Science (JSPS), KAKENHI Grant Number JP 20K09316 (T.H.), and JP 22H03178 (S.S.).


