Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Sandra López Fernández1, Carmen Chicharro2, Javier Nieto2, Fernando Fariñas Guerrero3, Eduardo Martínez Manzanares1,4 and Encarnación Clavijo Frutos1,4
Received: March 27, 2023; Published: April 10, 2023
*Corresponding author: Sandra López Fernández, Department Medical Microbiology, Faculty of Medicine, University of Malaga, Calle Louis Pasteur 32, 29010 Malaga, Spain
DOI: 10.26717/BJSTR.2023.49.007835
Diptera spp. and Ixodidae spp. can transmit several diseases through their bite, a lot of them are vectorborne
zoonoses that can affect humans who live near dogs. It is knowledge that isolation of reservoirs of
vector-borne diseases helps to presser the transmission and down the prevalence on the diseases. During
the epidemy of SARS-COVID-19 confinement in Spain, owners and dogs were isolated into their homes,
reducing the Potential reservoirs for VBD and giving an awesome opportunity to check how this hypothesis
is true for VBD. Sample of 3135 dogs were tested along 7 years with Quantitative Immunoassay (IFA,
ELISA) and PCR test to detect antibodies and pathogen of main Mediterraneum VBD (Leishmania infantum,
Anaplasma phagocytophilum, Dirofilaria immitis, Babesia canis and Ehrlichia canis), health check, blood test
(hemogram, biochemical, proteinogram).
Results were separated into 2 populations, pre-pandemic tested vs pandemic tested and compared both
groups. We found that the levels of positive dog’s pre-pandemic group were much higher than pandemic
levels.
This study shows that isolation of positive dogs far to healthy dogs and vectors help to low risk of contagious
for other dogs and, most importantly, Humans. This information can help to preserve Public Health and
avoid contagion.
Keywords: Leishmaniasis; Dirofilariasis; Anaplasmosis; Babesiosis; Piroplasm; Ehrlichiosis; Vector Borne Diseases; Mediterraneum Vectorial Zoonosis; Sars-Covid-19 Confinemen
Five Vector-Borne Diseases (VBD) zoonosis agent are the most common in Mediterraneum [1] dogs population, two bacteria (Ehrlichia canis and Anaplasma phagocytophilum), one nematode (Dirofilaria immitis) and two protozoans (Babesia canis, Leishm ania infantum). E. canis is transmitted transstadially and intrastadially only by ticks known “Brown Dog tick” [2,3] Rhipicephalus sanguineus complex. A. phagocytophilum has only one vector, Ixodes Ricinus [4,5] but Ehrlichia spp. Has been found in I. persulcatus [6], but not in Spain by now. Both bacteria infect the tick through the feeding [7] and can infect new subjects when ticks molt and bite again a new host or pass to a new tick generation by trans-ovarian transmission. Dirofilaria immitis is one nematode transmitted by several species [8] of mosquitoes [9] from genera Culex, Aedes, Anopheles and Ochlerotatus. All filaria have filiform elongated body.
Females produce embryos, the microfilaria, who swim in vascular system and go to the Diptera mosquitoes bite the vertebrate carrier and act like secondary host, developing the third stage of the larvae. 1 or 2 weeks after the bite, the new bite transmits the larvae to a new host, human or animal. Under the name Babesia canis[10,11] there are three related subspecies of this Genus, B. canis canis[12], B. canis rossi, and B. canis vogeli. They are grouped known as “Large Piroplasmids”. Neither can be identified under microscope observation, the three subspecies share similar external antigens, so immunoassays do not distinguish from each other, being necessary PCR test to identify each one. Truly, Clinical difference is not important for treatment, but it is a main factor by epidemiologist studies. All of them are transmitted by the “Hard-Ticks” Dermacentor reticulatus [13,14]. The female tick bites one carrier host and can transmit the protozoa to a new host after the molting or directly pass the new tick generation though trans-ovary transmission, in this way, new immature tick can carry the parasites since first stage.
At least, Leishmania infantum is a flagellated protozoa transmitted by sandflies in Spain, it has high incidence [15] in Mediterraneum coast and is a neglected [16,17] zoonosis includes in the EDO [18] list (obligatory declaration list) in Spain. A lot of dogs are infected by this parasite displaying symptoms or not [19]. One sandfly female [20] feeds on a carrier vertebrate, eating amastigote form of L. infantum. The parasite changes to a promastigote form, stretching the flagella, and goes to cranial intestine where begging to multiplicate between intestinal microvilli. New leishmanias migrate to the head where finish their maturation, and then, flagellum Metacyclic Promastigote can be inoculated in a new host though sandfly bite 2 weeks after infection. At the beginning of SARS-COVID-19, Spanish government declared the state of health alert [21] prohibiting the exit of people from their homes except in exceptional cases. Because of this, dogs were isolated at home with their owners providing an exceptional opportunity to study how dogs’ isolation affects vector-borne diseases transmission.
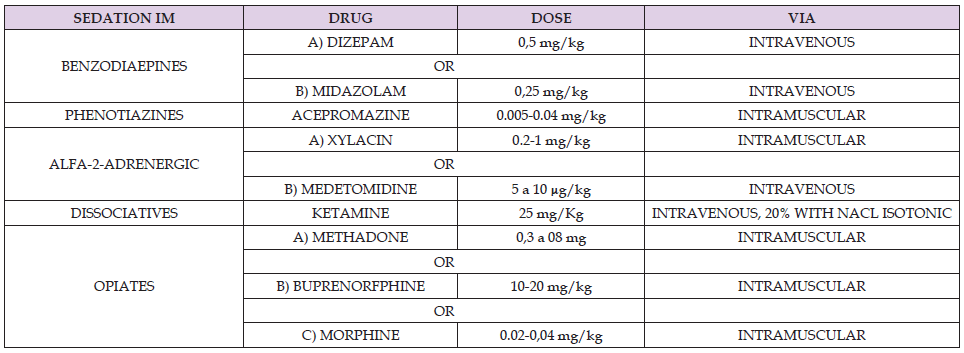
This was a retrospective observational study in 1320 dogs that were selected from the database (5000 initial subjects) of a previous epidemiological survey which sought to explore the distribution of canine vector-borne zoonoses in South of Spain. from 3135 dogs checked in Shelters, Pounds, and clinics from Malaga we selected dog from homes left in pounds or shelters and dogs from veterinary clinics and all tests were done under sedation (Table 2).
Different variables were collected from each subject (Table1) samples selected were:
1) Blood: Three sets were separated, one set for Málaga University (UMA), one set for Health Institute Carlos III (ISCIII) in Madrid and other set for reference laboratory.
The blood was separated into three tubes for each set, one Heparin-Li, one EDTA and one silicone gel. As well one drop was affixed onto tree slides to check microfilaria larvae and other parasite forms. From the UMA copy gel tube, another 4 more drops were collected to make Lateral-flow test (quick rapid antibodies test [22]). Serum and blood were sent to reference laboratory to issue for the performance of different tests (Table 1).
Table 1. DIAGNOSTICAL TEST: blood and tissues where collected and tested in the same day or processed and freeze at -20ºC (not blood).
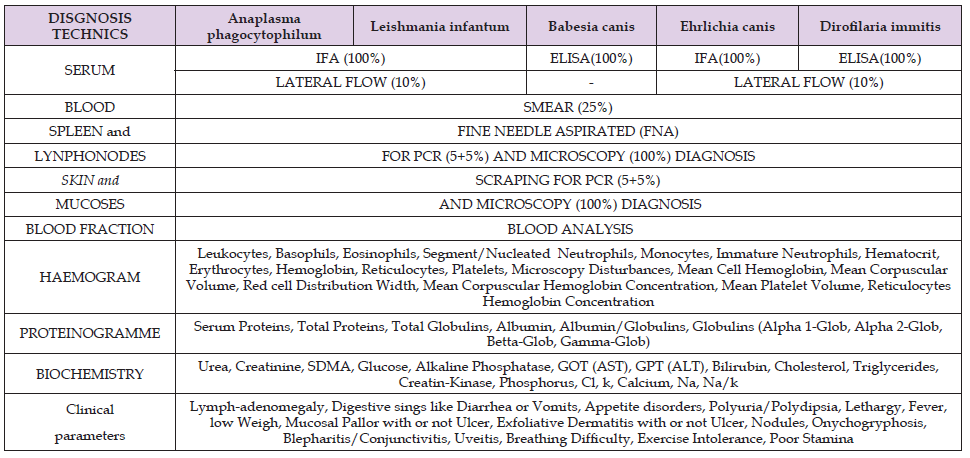
Table 2. Sedative drugs used to collect samples. Chemical Drug name are add with the empirical doses and were injected intra-muscular way.
The blood was separated into three tubes for each set, one Heparin-Li, one EDTA and one silicone gel. As well one drop was affixed onto tree slides to check microfilaria larvae and other parasite forms. From the UMA copy gel tube, another 4 more drops were collected to make Lateral-flow test (quick rapid antibodies test [22]). Serum and blood were sent to reference laboratory to issue for the performance of different tests (Table 1).
2) Tissues: Three sets were collected as well, for UMA, ISCIII
and reference Laboratory. The tissues selected were:
a) Bone marrow form right Rib-Cartilage edge, by FNA
technique (Table 1) with 21G needle.
b) Lymph-node from popliteus gland, by FNA technique (Table
1) with 21G needle.
c) Mucous Membrane from lips, by Scraping technique (Table
1), with nº 20 Surgical Blade.
d) Skin from internal side of ears, by Scraping technique (Table
1), with nº 20 Surgical Blade.
Samples were entered in a tube with Tris-EDTA buffer solution [23] until arrival at the university laboratory, where were homogenized and DNA purified with commercial kit [24] and freeze at -20ºC before to send to ISCIII and the other laboratories to keep at -80ºC. DNA sets were tested with PCR for L. infantum [25] in ISCIII and B. canis [26] in Reference laboratory [27]. 5% of the Spleen, lymph-nodes, mucosa, and skin sampled collected, and mucosa for microscope observation (Commercial [28] Diff-Quick stain). D. immitis [29] was checked from blood samples, all with ELISA and Lateral flow techniques and 25% was checked using simple microscopy technique, one drop of blood adds with one drop of sterile NaCl 9% physiological [30] serum and observed directly. An additional 5% were checked with MSD parasite diagnoses. The data obtained were listed in the statistical Table and analyzed with Fisher test and also submitted to the analyses of Correlation of Pearson.
A. phagocytophilum did not get carriers in 2016, as well E. canis in 2018 and 2022. The parasite most common was L. infantum all the years and D. immitis was the least represented. A. phagocytophilum and E. canis obtained similar results (Table 3). Tick borne bacteria A. phagocytophilum showed a decrease of -5.4% from Pre-pandemic (Graph 1) to Pandemic years while it in the other bacteria, E. canis, was -14.6% of decrease. The rate of A. phagocytophilum is smaller than E. canis based on E. canis of average prevalence is 18,8% in Prepandemic and decreases to 4.8% while A. phagocytophilum begins with 13,4% and decreases only to 8,0%. A similar process occurs if we compare B. canis front of A. phagocytophilum (Graph 1), being B. canis rate -7.5% of decreasing, 22,5% Pre-pandemic to 15,0% of Pandemic years. Mosquitoes borne diseases, D. immitis virtually has remained essentially unchanged, being the only parasite who shows a slight increase (Graph 1), 0.37%. It begins from 1,6% Pre-pandemic and climbs to just to 2,0% in Pandemic. However, L. infantum persists over time high prevalence levels (Graph 1), starting with 67,7% Pre-pandemic and plunging -35.04% of incidence until the 32,6% in Pandemic. Excluding D. immitis, the remaining diseases showed a significant reduction since Pandemic Isolated period as we can see in (Graph 2). Faced with more than one diagnosis technique, based on an image (Figure 1), laboratorial alterations (Figure 2), Clinical signs (Figure 3) and Serology (Figure 4). On an additional basis, Blood and tissues were tested by PCR technique to verification the laboratory results (Figure 5).
There are several possible conclusions to be drawn from the results obtained. First of all is the transmission of vector-borne diseases can be controlled protecting dogs from the vector, whether isolating (thus not feasible) or keeping out dogs from vector and this can be achieved using repellent substances for ticks and mosquitoes using collar or spot-on for example. If the vector cannot access the dog, the transmission is severely disrupted. The geographical area of South of Spain shows an important presence of vectors, mosquitoes, sandflies and ticks infected with infectious agents, so humans should be protected from vector. In the case of mosquitoes and sandflies, using Chemical Repellents (at evening outs), mosquito-nets on doors and windows, Electrical Devices Repelling at home (that provide extra-protection to the dogs). About tick, using high-top shoes and protective clothing in trips to the country, gardens, or parks, sprayed onto clothes repellent substances.
Adults must comprehensively review the body of kids and elderly persons after walks in the countryside. property garden should be often sprayed to kill adults and eggs of vector. The parents should be careful and teach their kids not to touch animals foreign. Relevant authorities are obliged to ensure the Public Health so, based on these results, they should take the necessary steps to protect population with actions like clean rivers, punctual fumigations, and follow the measures proposed by WHO [31] and OIE [32,33].
FIMABIS, Andalusian Public Foundation for Research in Biomedicine and Health of Malaga; IBIMA, Biomedical Research Institute of Malaga


