Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Shu-Lan Hao1, Peng Nan2, Zhi-Jie Wang1, Fu-Peng Zhang1, Hai-Fu Chen1, Yi-Zhe Zhang1, Wei Suo1, Li-Fang Yang3, Ge-Hong Zhang4, Li-Kun Liu1* and Xi-Xing Wang1*
Received: December 30, 2022; Published: April 06, 2023
*Corresponding author: Xi-Xing Wang, Department of Oncology, Shanxi Province Hospital of Traditional Chinese Medicine Address: No. 46 Bingzhou East Street, Yingze District, Taiyuan City, Shanxi Province, China
Li-Kun Liu, Department of Oncology, Shanxi Province Hospital of Traditional Chinese Medicine Address: No. 46 Bingzhou East Street, Yingze District, Taiyuan City, Shanxi Province, Chinat
DOI: 10.26717/BJSTR.2023.49.007830
Introduction: Colorectal cancer is the third most common malignancy and the fourth leading cause of death, among all malignant tumours. Patients with early-stage colorectal cancer, especially stage I and stage II patients with risk factors (for example, positive lymph node metastasis, vascular tumor thrombus, positive incision margins), require adjuvant chemotherapy after surgery to prevent recurrence and metastasis. Patients often experience fatigue because of the side effects of chemotherapy. Fatigue causes significant distress to the patient and affects the continuation of chemotherapy and may cause tumour recurrence and/or metastasis. Chinese herbal medicine can alleviate the degree of chemotherapy-related fatigue and improve the quality of life of the patient, thus enhancing their immunity.
Methods and Analysis: We intend to recruit 90 patients the control group will comprise 45 patients that are treated by XELOX chemotherapy. The treatment group will include 45 patients on XELOX chemotherapy and Yiqi Chupi powder. The primary outcomes are the score of the Piper Fatigue Scale (PFS); the secondary outcomes include disease-free survival (DFS), Karnofsky Performance Status (KPS) grade, score of traditional Social Sciences Chinese medicine syndrome, tumour markers and C-reactive protein. Security indicators include routine blood analyses, urinalysis, liver and kidney function tests, electrocardiographic examination and adverse events. All data in this randomised controlled trial will be analysed using the Statistical Package for the Social Sciences 26.0 (SPSS 26.0) software.
Ethics and Dissemination: This randomised controlled trial has been approved by the Human Research Ethics Committee of Shanxi Province Hospital of Traditional Chinese Medicine (2021-07020). Written informed consent will be taken from all patients prior to their participation, and they will be free to withdraw participation in this study at any time. Results of this study will be published in a peer-reviewed journal.
Registration: This randomised controlled trial has been registered on Chinese Clinical Trial Registry (ChiCTR2200057546).
Abbreviations: CRF: Cancer-Related Fatigue; NCCN: National Comprehensive Cancer Network; TNF: Tumour Necrosis Factors; BMI: Body Mass Index; TCM: Traditional Chinese Medicine; AJCC: American Joint Committee on Cancer; AST: Aspartate Aminotransferase; TBIL: Total Bilirubin; CR: Serum Creatinine; WBC: White Blood Cell; HB: Haemoglobin; PLT: platelet; SAS: Statistical Analysis System; PFS: Piper Fatigue Score; DFS: Disease-Free Survival
Strengths: By using Yiqi Chupi San to treat fatigue caused by postoperative chemotherapy for colorectal cancer, we can observe the effect of traditional Chinese medicine in chemotherapy, and hope to reduce the fatigue caused by chemotherapy and improve the quality of life of patients.
Limitations: Fatigue symptoms can occur in various stages of colorectal cancer, such as fatigue after surgery, fatigue after radiotherapy, and fatigue in advanced cachexia. The reasons for fatigue and the treatment measures vary. This article only analyses the fatigue caused by postoperative adjuvant chemotherapy, and does not include fatigue after surgery, fatigue after radiotherapy, and fatigue in advanced cachexia. The next step will continue to expand the sample size and observe the effect of Yiqi Chupi powder in different populations with colorectal cancer.
Colorectal cancer is the third most common malignancy worldwide. It is also the fourth leading cause of death among all malignant tumours. The treatment modalities for colorectal cancer include surgery, chemotherapy, radiotherapy, biotargeted therapy, gene therapy and biological immunotherapy [1,2]. However, due to the toxic side effects of chemotherapy, postoperative adjuvant chemotherapy damages the balance of the human body; patients often experience nausea, trichomadesis, fatigue and other adverse symptoms during chemotherapy, which seriously impacts the patients’ quality of life. Chemotherapy-related fatigue refers to the fatigue caused by chemotherapy, which is a sub-category of cancerrelated fatigue. The National Comprehensive Cancer Network (NCCN) Fatigue Domain Expert Group defines cancer-related fatigue (CRF) as ‘a persistent subjective feeling of fatigue that is associated with cancer or cancer treatment and not with recent activity, and it interferes with normal life’. Compared to the fatigue experienced by a healthy human being, chemotherapy-related fatigue is more severe, and cannot be alleviated by rest [3]. The aetiology of CRF has not been definitively defined; however, studies suggest that CRF is related to tumour occurrence, growth, treatment and psychological factors of patients themselves. Tumour cells can affect the function and metabolism of normal cells and promote the growth of tumour cells by releasing cytokines such as interleukin-2 (IL-2), tumour necrosis factors (TNF) and other cytofactors, which leads to reduction of body function and occurrence of fatigue [4]. Studies have shown that chemotherapy can cause a loss of appetite in patients, which further leads to a loss of skeletal muscle mass and a decrease in body mass index (BMI), which cannot be relieved by traditional nutritional support; this naturally leads to extreme fatigue in cancer patients [5].
Chemotherapy-related fatigue can impair the quality of life and potentially reduce overall survival by reducing a patient’s ability to complete cancer drug treatment and participate in essential and valuable life activities [6]. Traditional Chinese Medicine (TCM) believes that blood carries qi, and surgery, as an invasive treatment method, consumes qi (The theory of traditional Chinese medicine believes that qi is a nutritious and subtle substance flowing in the human body. It is often used to express the activity ability of zangfu organs clinically) and can dissipate qi through excessive bleeding, thereby damaging the vitality of the human body. Study had shown that chemotherapy can reduce the level of hemoglobin in the body’s blood, causing fatigue. Meanwhile, all patients who received chemotherapy had the most severe fatigue at 6 months [7]. Fatigue due to chemotherapy is the most common form of CRF. Chemotherapy drugs also kill the healthy tissue cells of the body; this results in energy consumption for the body’s repair, thus aggravating fatigue. In addition, myelosuppression and gastrointestinal symptoms caused by chemotherapy drugs can also exacerbate fatigue [4].
TCM believes that fatigue is caused by overexertion and congenital physical weakness, acquired disease factors, overworking and improper diet. Many scholars believe that the main aetiology of ‘xulao’ is the deficiency of visceral function and the deficiency of qi, blood, Yin and Yang. Its clinical manifestations are mental exhaustion, dyspnoea, insomnia or lethargy, inattention, cognitive decline, depression, among other symptoms. Further, the theory of TCM believes that tumour incidence is based on that the weakness of the body as the foundation, and pathogenic factors is the standard. Weakness is the patients’ cause of cancer. Owing to tumours and chemotherapy drug reactions on the body, patients will develop symptoms of deficiency of visceral function, qi and blood, yin and yang during the chemotherapy process, it will take an extended amount of time to achieve recovery from these symptoms [8]. Currently, there are few studies on fatigue caused by chemotherapy in CRC patients, but the existing studies have shown that fatigue gradually increases with the progress of chemotherapy, and the degree of fatigue is more severe and lasts longer than that in patients who do not receive chemotherapy [7,9]. Western medicine mainly uses central stimulants, antidepressants, corticosteroids and other symptomatic treatments. These drugs, along with timely albumin and blood transfusions, can relieve the pain and fatigue of the patient in a short time, and improve the mental state of the patient. Chinese medicine treats fatigue through the compatibility of various nourishing Chinese medicines to enhance immunity. YQCP is a formula to treat the fatigue of qi deficiency and kidney deficiency. It is developed by Professor Xixing Wang, a master of traditional Chinese medicine.
It consists of raw Astragalus, Codonopsis, Ophiopogon japonicus, Schisandra, Cimicifuga, Radix Scutellariae, and dogwood meat. Studies have shown that astragalus is rich in active substances, among which astragalus saponins can inhibit the growth and proliferation of colorectal cancer cells, and can also promote the apoptosis of cancer cells. Flavonoids have immune-boosting and antioxidant properties [10,11]. Codonopsis polysaccharides in Codonopsis can activate the immune system, improve symptoms of fatigue, and can also fight tumors [12]. Ophiopogon saponins D and Ophiopogon B have beeAngelica sinensisn shown to have antioxidant and anti-tumor properties by inhibiting tumor cell metastasis [13,14]. Schisandra chinensis supplement extract can increase human muscle strength [15]. Cimicifuga extract can significantly improve fatigue caused by decreased estrogen levels [16]. AS has anti-fatigue activity by reducing serum lactate and ammonia levels, increasing hepatic and muscle glycogen deposition, [17] Baicalin in Scutellaria baicalensis can be anti-oxidative and anti-tumor [18]. Professor Xixing Wang follows the principle of «benefiting those loss» and «treating deficiency with tonification” and adopts traditional Chinese medicines that can strengthen the spleen and qi, and nourish the lungs and kidneys, so that the patient’s middle energizer, spleen and stomach can be restored, qi and blood biochemically active, and finally achieve the state of coordination of viscera, yin is at peace and yang is compact. We will conduct this study to further verify whetherYiqi Chupi powder can significantly improve chemotherapy-related fatigue, enhance patient immunity, improve patient quality of life, and further increase the disease-free progression period of patients.
Study Design
This multicentre randomised controlled clinical trial will assess the efficacy of Yiqi Chupi powder in the treatment of chemotherapyrelated fatigue post-colorectal cancer surgery. The included patients will be divided into two groups: the treatment group and control group. The patients will be followed up for two years after the initial 6-month treatment period. We will collect patients requiring adjuvant chemotherapy after colorectal cancer surgery from December 2021 to December 2022, then filtering these patients according to inclusion and exclusion criteria. Eligible patients will sign informed consent and then undergo randomisation. The control group will comprise 45 patients that are treated only by XELOX chemotherapy (a combination of oxaliplatin and Xeloda (capecitabine): oxaliplatin 130 mg/m2, administered by intravenous injection on the first day of the chemotherapy cycle, capecitabine at 1000 mg/m2 on the first day to the 14th day of the chemotherapy cycle, orally twice a day, continuously taken, suspended for one week, for a cycle of 21 days). The treatment group will include 45 patients on XELOX chemotherapy as well as Yiqi Chupi powder. Data collection and analyses will be conducted, followed by writing of the final paper for publication. The flow diagram is shown in the (Figure 1).
Patient and Public Involvement
No patient involved.
Patients
Diagnostic Basis: Referring to the New Code for Diagnosis and Treatment of Common Malignancies written by the Chinese Anti- Cancer Society, all cases must be clearly diagnosed as colorectal cancer by pathology or cytology. Postoperative staging of colorectal cancer has been done in accordance with the American Joint Committee on Cancer (AJCC) / International Alliance Against Cancer (UICC) TNM staging of colorectal cancer (8th edition). Diagnosis standard of TCM refers to the textbook of TCM Diagnostics (2007) published by China Traditional Chinese Medicine Press, and Modern Traditional Chinese Medicine Oncology (2004) published by Shanghai Traditional Chinese Medicine Press. Diagnostic criteria of qi deficiency and kidney deficiency after colorectal cancer surgery was formulated by referring to the 2002 trial version of the Guiding Principles for Clinical Research on New Chinese Medicines.
Inclusion Criteria
1. The pathological examination conforms to the diagnostic criteria of Western medicine for colorectal cancer.
2. Postoperative stage of colorectal cancer is stage II with highrisk factors (positive lymph node metastasis, vascular tumour thrombus, incisal edge positive, etc.); or postoperative stage is stage III and requires postoperative adjuvant chemotherapy.
3. Complies with the diagnostic criteria of TCM syndrome of qi deficiency and kidney deficiency.
4. Patient’s age is between 18-80 years old.
5. No sex or gender restrictions.
6. Liver and kidney function and haematopoietic function are normal (in the absence of on-going supportive care).
7. Expected survival ≥ 6 months.
8. The score of Karnofsky Performance Status (KPS) ≥60 points.
9. Signing the informed consent voluntarily.
Exclusion Criteria
1. Patients with mental disorders (mental disorders or severe dementia).
2. Abnormal liver and kidney functions (aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL) values higher than 2.5 times the upper limit of normal values or blood urea nitrogen ( BUN) and serum creatinine (Cr) levels higher than 2 times the upper limit of normal values), abnormalities in cardiac enzyme levels (CK or CK-MB values above the normal upper limit); Abnormalities in routine blood test results (white blood cell (WBC) counts, haemoglobin (Hb) levels, platelet (PLT) counts are lower than normal).
3. Patients who also have other serious diseases of the circulatory system, haematopoietic system, digestive system, endocrine system, or other diseases.
4. Refusing postoperative adjuvant chemotherapy.
5. Pregnant/breastfeeding women or women who intend to conceive.
6. Patients who participated in other clinical research studies in the past month.
Shedding Criteria
1. Those found to be inconsistent with the inclusion criteria after inclusion.
2. Patients with poor compliance who fail to take medicine and review the indicators as required.
3. Use of other TCM besides Yiqi Chupi powder.
4. No medication or no record after medicating.
5. Not reporting for follow-up and/or withdrawal from clinical investigators due to various reasons.
Sample Size: Sample size estimation formula:
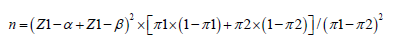
α is taken as 0.05 on one side; β is taken as 0.1;
π0: Effectiveness of the drug in a similar study: 80%;
π1: Estimated effective rate of Yiqi Chupi powder: 95%;
After calculation, N≈79. Therefore, the total number of samples is 79. Considering dropout factors and operability, 45 patients will be included in the control group and 45 patients in the treatment group.
Randomization:
The patients will be sorted from 1 to 90 according to the order of inclusion, and Statistical Analysis System (SAS) software will be used to generate random numbers. Odd numbers will be included in the treatment group, and even numbers will be included in the control group. If the treatment group meets 45 cases first, the rest of the patients will be assigned to the control group and vice versa.
Outcomes
Primary Outcomes: PFS (Piper Fatigue Score): The degree of fatigue will be assessed by the Piper Fatigue Self-assessment Scale (PFS) before and after treatment. There are four dimensions, including behaviour (6 items, 22 entries), emotion (5 items), perception (5 items), and cognition (6 items). Each dimension is 0-10 points; the PFS will be calculated from the average of the four dimensions. PFS values of 0 to 3.3 points are classified as mild fatigue, 3.3 to 6.7 points are classified as moderate fatigue, and 6.7 to 10 points are classified as severe fatigue.
Secondary Outcomes
1. Disease-Free Survival (DFS): Time from randomisation to disease recurrence or patient death due to disease progression.
2. TCM Syndrome Scores: Scoring 0, 1, 2, and 3 according to none, mild, moderate and severe, respectively, and recording fatigue, shortness of breath, lazy speech, poor appetite, pale complexion, weak waist and knees, pale and fat tongue, presence of thin and white coating on the tongue, weakness and pulse. The curative effect is determined according to the integral method and the curative effect index: If clinical symptoms disappear or mostly disappear, the syndrome integral is reduced by ≥95% which designates cure; If the clinical symptoms improve significantly, the syndrome integral is reduced between 70% and 90%, which indicates significantly effective; If all clinical symptoms have improved, the syndrome points are reduced between 30% and 70%, designating effective; If there is no significant improvement in clinical symptoms, the syndrome points are reduced by <30%, meaning invalid.
The therapeutic index (n) = (total pre-treatment points - total post-treatment points) ÷ total pre-treatment points × 100%.
3. KPS: The higher the score, the better the health condition, and the more tolerable the treatment. If the score after treatment is more than or equal to an increase or decrease of 10 points from before treatment, this means the treatment is effective. An increase or decrease of less than 10 points in the score after treatment means stable condition.
4. Changes in the levels of gastrointestinal tumour markers, and peripheral blood C-reactive protein levels will be examined.
Safety Outcomes: Routine blood and urine tests, kidney and liver function tests, electrocardiogram examination and adverse events.
Data Management and Quality Control:
Any changes to the protocol will be approved by the ethics committee of Shanxi Province Hospital of TCM. All patient data will be recorded in case report form by a trained researcher. Patients’ information will not be shared with any third party without their permission.
Statistical Analysis:
All data will be recorded in detail; it will be scored and reviewed by special investigators. The results will be recorded in Microsoft Excel 2019 to establish a database. The statistical data will be analysed by SPSS 26.0 software. The comparison between count data will be performed by chi-square test, measurement data will be expressed by mean ± standard deviation, t-test for normal distribution, and rank-sum test for non-normal distribution; a value of P < 0.05 will be statistically significant.
Ethics and Dissemination:
This randomised controlled trial has been approved by the Human Research Ethics Committee of Shanxi Province Hospital of Traditional Chinese medicine (2021-07020). Written informed consent will be taken from all patients prior to their participation, and they will be free to withdraw participation from this study at any time. Results of this study will be published in a peer-reviewed journal.
Registration:
This randomised controlled trial has been registered on the Chinese Clinical Trial Registry (ChiCTR2200057546).
Conceptualisation: Xi-Xing Wang and Li-Kun Liu.
Data Curation: Nan-peng, Ge-Hong Zhang and Li-Fang Yang.
Methodology: Zhi-Jie Wang and Fu-Peng Zhang.
Data Analysis: Zhi-Jie Wang.
Supervision: Li-Kun Liu and Shu-Lan Hao.
Writing-Original Draft: Shu-Lan Hao and Peng-Nan.
Writing-Review and Editing: Shu-Lan Hao, Peng-Nan, Zhi-Jie Wang, Fu-Peng Zhang, Hai-Fu Chen, Yi-Zhe Zhang, Wei Suo, Ge-Hong Zhang, Li-Fang Yang, Li-Kun Liu and Xi-Xing Wang.
This work was supported by Shanxi Traditional Chinese Medicine Clinical Research Center (LCYJZX202109) and Scientific Research Project of Shanxi Provincial Health Commission (2020XM04) (2020TD04).
There are no competing interests for any author.


