Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Marakhouski Y*, Zharskaya O and Karasiova G
Received: March 17, 2023; Published: April 05, 2023
*Corresponding author: Marakhouski Y, Belarusian Medical Academy of Postgraduate Education, Minsk, Belarus
DOI: 10.26717/BJSTR.2023.49.007824
The study results showed the high efficiency of the combination in one form of two active substancesalverine (alverine) and simeticone (simeticone) – in the drug Meteospasmyl according to the results of self-assessment by patients with overlapping symptoms of uninvestigated dyspepsia(UD) and nonspecific irritable bowel syndrome(IBS-N), with a positive effect of 86.7% (95% CI Fisher’s 78.6–92.5) cases and a high Odds ratio (chance) (22.7, at 95% CI 8.9–58.4) of the effect compared with the comparator group (treatment without the use of Meteospasmyl). Treatment adherence higher in 12 times (Odds ratio) (95% CI 1.42– 112.06) with Meteospasmyl (85.7% (95% CI 63.7–97.0)), versus the comparator group (33.3% (95% CI 7.5–70.1)). Thus, Meteospasmyl can be used as a first-line drug in overlapping dyspepsia with an nonspecific variant of irritable bowel syndrome, especially during the period of performing and waiting for the results of the patient’s clarifying examination. A high safety degree of this drug has been demonstrated, both in terms of clinical and laboratory parameters. Results were obtained confirming the effect of Meteospasmyl on skeletal muscles with a significant increase in the grip strength of the right hand both at the 2nd week of treatment with Meteospasmyl (increase by 0.4 kg) and by the 4th week of treatment (by 0.6 kg), in contrast with comparison group (F-test ANOVA (2; 60) = 7.2; p=0.0015; Kruskal – Wallis test-H (2; 63) = 27.6161; p=0.00001). The applied method of multivariate analysis of variance with a hypothesis decomposition model confirmed the effect of Meteospasmyl on the increase in the values of hand dynamometry on the 28th day of admission (p trend less than 0.001).The advantages of the presented study are the following characteristics:
1. New data were obtained on the option of overlapping uninvestigated dyspepsia with nonspecific
irritable bowel syndrome.
2. Patients’ self-assessment of effectiveness in this variant of disorders was not previously studied.
3. Meteospasmyl demonstrated greater efficiency in comparison with mebeverine and drotaverin;
4. Meteospasmyl increased the functional state of skeletal muscles.
5. Meteospasmyl showed a high safety profile.
Keywords: Meteospasmyl; Uninvestigated Dyspepsia; Nonspecific Irritable Bowel Syndrome; Symptom Overlap; Efficacy; Safety
Abbreviations: FGID: Functional Gastrointestinal Disorders; FD: Functional Dyspepsia (FD); IBS: Irritable Bowel Syndrome; SDM: Shared Decision-Making; PROs: Patient-Reported Outcomes; LEC: Local Ethics Committee; UEG: United European Gastroenterology; UD: Uninvestigated Dyspepsia; TB: Total Bilirubin; CB: Conjugated Bilirubin; TC: Total Cholesterol
At present, the Rome V criteria (Rome V, Disorders of Gut-Brain Interaction 5th Edition) are being developed, with an emphasis on the key aspects for revision of a number of provisions, including elimination of diagnostic problems in the overlap syndrome (Address Diagnostic Overlap). In the Russian-language literature, this group of disorders (overlap syndrome) has several designations: “combined functional gastrointestinal disorders» (SFGID),»crossover syndrome», «comorbid functional disorders» [1]. However, nether common designation, nor general international classification for this group of disorders is available so far, which causes significant practical problems in differential assessment of the functional digestive system disorders overlap syndrome. On the other hand, there is a practical clinical significance of functional gastrointestinal disorders (FGID), since the presence of the “overlap syndrome” worsens the course of and treatment outcomes in such conditions [2,3]. And the most common symptoms overlap is the combination of functional dyspepsia (FD) and irritable bowel syndrome (IBS). Thus, it is necessary to develop a strategy for improving accuracy of overlap syndrome diagnosis, efficacy and safety of drug treatment in such disorders. Further, in terms of psychological differences of such patients, patient-oriented assessment of treatment efficacy and safety, including shared decision-making (SDM) and patient-reported outcomes (PROs) if possible, is important.
Efficacy and safety clarification of alverin/simethicone combination administration in overlap syndrome of dyspepsia and bowell-related symptoms by available in real clinical practice indicators and patients’ self-assessment
General Study Design
The study was conducted in accordance with the regulatory requirements of the Republic of Belarus and complied with the scientific purpose and importance and was carried out within the common research practice described in the following documents:
1. Declaration of Helsinki 2008.
2. ICH E6(R1): Guideline for Good -Clinical Practice, version
4, International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. The study protocol is approved by the Local Ethics Committee (LEK). All study participants signed an informed consent to participate. This study was designed as a parallel comparative two-arm study: the investigational medicinal product (alverin with simethicone - test group A) compared with a combination of medicinal products - simethicone with an antispasmodic (mebeverine or drotaverine) (comparator - control group B). The study subjects had no previous history and/or current diagnosis of manifestations suggesting a clinically significant pathology of systems and organs. The presence of dyspepsia symptoms combined with symptoms suggesting bowell disorders was allowed. Source data were recorded in a paper-based case report form (CRF). Description of the method of study depending on selection and formation of groups of study subjects. The study protocol is approved by the Local Ethics Committee (LEK). The general study design is presented in Figure 1. Based on the inclusion/ exclusion criteria, 30 out of 75 patients examined were included in the study. For additional control, prognostic adequacy of the number of follow-ups was assessed, using the Win Pepi [4] section - group size determination (Sample size a difference between proportion) according to the predicted efficiency difference (A-80%, B-27%) with a reliability of 0.05 and a power of 80%, with the ratio of A / B = 2. The obtained results are as follows: Required sample: Total 30 (20 in A, 10 in B), Expected precision: Approx. 95% CI for difference between proportions (D) = D – 0.402 to D + 0.402. 3 visits were made with a follow-up examination: Visit 1 - initial (before initiation of treatment), Visit 2 - follow-up with an examination after 14 days of treatment, Visit 3 - final (after 28 days of treatment). An additional assessment was performed 2 weeks after the end of treatment by free telephone interview.
Table 1. Distribution for randomization.
Note: In total - group A - 20 subjects, group B - 10 subjects.
Study Subjects
Group A. Height: average 169.9 (95% CI 165.2–174.5), median 167 (Q-25–Q-75 = 164–175); weight: average 77.8 (95% CI 69.5- 86.1), median 77 (Q-25–Q-75 = 62–86); age: 46.5 (95% CI 39.7–53.4), median 45 (Q-25–Q-75 = 31–60).
Group A comprised 23.8% (95% CI (Fisher’s) 11.3–52.2%) of males. 5 volunteers in this group were smokers, which is 23.8% (95% CI (Fisher’s) 8.2-47.2%).
Group B. Height: average 170.0 (95% CI 164–176), median 172 (Q-25–Q-75 = 164-173); weight: average 71.9 (95% CI 60.2–83.7), median 71 (Q-25–Q-75 = 62–80); age: 45.4 (95% CI 35.0–55.9), median 49 (Q-25–Q-75 = 37–53).
Group B comprised 11.1% (95% CI (Fisher’s) 0.3–48.2%) of males. Group B had 1 smoker, which is 11.1% (95% CI (Fisher’s) 0.3– 48.2%).
Comparison of the analysis results in groups A and B showed that there was no¬ statistically significant difference in height, weight and age. There is a difference in the number of smokers: group A had a larger number of smokers. Consumption of alcoholic beverages was analyzed according to the criteria set out in publications [5,6] and allowing to assess the situation in terms of the following characteristics: nondrinkers, light drinkers, moderate drinkers and abusers. Results obtained: drinking alcohol several times per month – 24.4% (95% CI 12.9-39.5), several times per year – 55.6% (95% CI 40.0-70.4), never drinking – 15.6% (95% CI 6.5-29.5); they characterize this group of volunteers as a group of¬ nondrinkers and light drinkers who do not exceed the tolerable level of consumption of alcoholic beverages. Drinking alcoholic beverages during the last 7 days before the first visit and throughout the study was not reported.
Specific of Methodology and Study Methods
The group for inclusion in the study was selected by questionnaire survey, subject to the basic Rome IV criteria. So, the following symptoms were¬ considered: epigastric pain and/or burning, early satiety, satiety after meals, discomfort (functional dyspepsia consensus of the United European Gastroenterology (UEG) and European Society of Neurogastroenterology¬ and Motility (ESNM) (1,2) [7]. Symptoms¬ were detailed according to the modified Leeds Dyspepsia¬ Questionnaire validated using more than 18,000 cases [8]. Actually, patients with uninvestigated dyspepsia (UD) with excluded alarm symptoms were selected. Additionally, a questionnaire according to basic Rome IV criteria¬ recommended for bowel diseases [9,10] was used. Patients with¬ intestinal symptoms associated with an organic cause or fully complying with the criteria for well differentiated forms of functional intestinal disorders (IBS with diarrhea, IBS with constipation, mixed IBS,¬ functional constipation and diarrhea) were excluded. To increase confidence in the absence¬ of intestinal pathology at the stage of randomization, fecal calprotectin test was performed. Actually, patients met Rome IV criteria for unspecified functional bowell disorder (IBS -nonspecific) and had symptoms for at least 3 months [11,12]. The following Rome IV criteria for nonspecific bowell disorder were met: 1 or more other key symptoms (abdominal pain, abdominal distention / bloating, constipation or diarrhea) were present at the minimum diagnostic yield threshold¬ for these disorders in the last 3 months, but there were no criteria for diagnosis of any specific bowel disorders [13].
Thus, general clinical profile of the subjects can be determined as patients with uninvestigated dyspepsia (UD)¬ overlapping with an unspecified functional nowell disorder in the form of nonspecific IBS (IBS-N). The clinically oriented (symptom-based) assessment used is positively characterized by the US National Institute for Clinical Excellence (NICE); NICE experts recommend that general practitioners¬ use a symptom-based approach in patient care [14], which is consistent with conclusions that predominantly symptomatology rather than endoscopic presentation is the best guide in assessing treatment response. Treatment efficacy assessment was based on patient-reported outcomes (PROs) obtained during the final¬ treatment visit (Visit 3). Effects were detailed according to a special¬ self-assessment questionnaire (medication and treatment satisfactory questionnaires-TSQ) validated using 18,724 cases in an earlier study [8]. Patient satisfaction with the effects was assessed based on the level of agreement (“Strongly¬ agree», «Agree») or disagreement («Disagree», «Strongly disagree»)¬ with 7 separate statements presented in Tables 2-4. Additionally, taking into account peculiarities of alverin action, the participants¬ of the study undergone a grip test.
To assess safety and acceptability of the treatment, a number of parameters available in routine clinical practice were determined during all visits: blood chemistry values, hematological blood values, urinalysis values. Biochemical analysis was performed by methods of quantification¬ of components in blood serum. The analysis was performed on biochemical¬ analyzers Dialab Autolyzer (Austria), FP- 901 (Finland) using¬ diagnostic kits SPINREACT (Spain), Analytik Jena AG (Germany). Blood serum chemistry parameters determined during the study were as follows: total bilirubin (TB) and conjugated bilirubin (CB), total cholesterol (TC), creatinine, uric acid, aspartate aminotransferase and alanine aminotransferase (AST, ALT), alkaline phosphatase (AP), pancreatic amylase. Serum TH concentration was determined by the¬ enzymatic Trinder method (1969). AST, ALT, AP enzyme activity was determined by kinetic methods. The highly sensitive C-reactive(hsCRP) blood protein was determined using a reagent kit for highly sensitive enzyme immunoassay of C-reactive protein concentration (CRP-ELISA-BEST highly¬ sensitive, No. RZN 2016/3872). During the randomization visit, hsCRP was determined in all patients; if values were below 5 mg/L, the absence of a systemic inflammatory response was reported and only such patients were included in the study. Fecal calprotectin was determined using Actim Calprotectin, a rapid test kit for semi-quantitative calprotectin assessment, with the cut-off value¬ 50 μg/L to exclude an inflammatory process in the bowell, and only patients¬ with values within the reference value were included in the study.
Statistical Methods
Statistical processing of the results was performed by means of Statistica-6 application package, version 6.1, series 1203d, WinPepi (2004), NCSS 2004 R program, using descriptive statistics methods, analysis of variance¬ on a personal computer. All data from the clinical study were checked for compliance with the Gaussian¬ distribution. For this purpose, we used the quantitative Shapiro-Wilk test (Shapiro- Wilk W), built quantile plots, compared the distribution histogram of the test parameter with the theoretical normal distribution curve at the estimated values of the mean and standard deviation. If a test value¬ significantly exceeded the significance level p=0.05 used as the critical level and no significant deviations from the straight line in quantile¬ plots were observed, it was considered that there were no grounds to reject the assumption that the test parameter corresponded to the Gaussian distribution. With test values close to the critical value, the decision on the distribution conformity was made based on the type of quantile plots. Additionally, the distribution normality¬ was assessed according to seven criteria: Shapiro – Wilk W, Anderson-Darling, Martinez-Iglewicz, Kolmogorov-Smirnov, D’Agostino Skewness, D’Agostino Kurtosis, D’Agostino Omnibus. The final decision on the distribution normality was made taking into account all the criteria, however, in case of different variants of values, two criteria were preferred: Shapiro - Wilk W and Kolmogorov - Smirnov.
If the distribution significantly deviated from the Gaussian distribution, an attempt was made to normalize the data using various reversible transformations: logarithmation¬, double logarithmation, calculation of reciprocal values. Distribution¬ of the obtained values was examined and a decision was made thereunder whether to use parametric or nonparametric methods of analysis in the future. If the distribution of the test quantitative parameter (or its transformation) corresponded to the Gaussian distribution, the data were presented as an arithmetic mean value with a confidence interval, and¬ standard deviation if necessary. Otherwise, data were presented as median and quartiles and/or percentiles.
Assessment of Treatment Efficacy Based on Self- Assessment by Patients
Questionnaire, item No. 1 “Please, rate the degree of symptoms relief which is achieved after Meteospasmyl treatment», showed the following results: 5 of 21 cases (23.8%, 95% CI (Fisher’s) 8.2-47.2) – moderate relief, 11 cases (52.4%, 95% CI (Fisher’s) 29.8-74.3)-significant relief, 5 cases (23.8%, 95% CI (Fisher’s) 8.2-47.2) -the symptoms were completely eliminated. Thus, a pronounced positive effect of Meteospasmyl treatment was noted by 76.2% (95% CI (Fisher’s) 52.8-91.8) of the study participants. Table 2 shows cumulative self-assessment results in group A (Meteospasmyl treatment) for other questionnaire items. As shown in Table 2, 95.2% (95% CI 76.2-99.9) of the patients were satisfied with the timing of onset of a Meteospasmyl positive effect; symptom reduction¬ was noted by 95.2% (95% CI 76.2–99.9) and health improvement¬ was noted by 85.7% (95% CI 63.7-97.0) of the study participants. Confidence that Meteospasmyl will continue to have a positive effect was noted by 81% (95% CI 58.1-94.6) of the participants, ease of administration was noted by 100%, intention to continue this medicinal product was noted by 85.7% (95% CI 63.7-97.0) of the respondents.
Thus, Meteospasmyl treatment resulted in a reliable symptoms reduction and was associated with a significant patient health improvement. Table 3 presents the results of self-assessment in the reference group (group B -reference group (comparator treatment)). Differences between group A and group B were compared using multifield frequency tables; the results are presented in Table 4. Chances of a positive treatment outcome in group A (Meteospasmyl) exceed the same chances in comparison with group B (other drugs) by Odds Ratio(OR): in terms of symptom relief – 11.2-fold (95% CI 1.4-131.2), in terms of timing of action-73.5-fold (95% CI 4.4-3456.4), in terms of satisfaction with the symptoms reduction -42.0-fold (95% CI 2.9-2017.7), in terms of confidence in the effect – 34,0-fold (95% CI 2.7-1593.0), in terms of intention to continue administration – 12.0-fold (95% CI 1.4–112.1). Cluster analysis of response rate in total for 5 (1–5) questions of the difference between groups A and B: Clusters: A = 5; B5 Observations (observations -N): A = 105; B = 45. «Yes» observations (positive response): A - 91; B-10. Rate of «Yes» observations (positive response rate): A-86.7% (95% CI Fisher’s 78.6–92.5) and B – 22.22% (95% CI Fisher’s 11.2-37.1). Reliability by the Donald – Donner method (method): Chi-square = 52.92 (1 df), P = 0.0001, Odds Ratio (OR) (A: B) = 22.7 (Approx. 95% CI 8.9-58.4).
Thus, the chance of a positive treatment outcome in total for items 1–5 of the self-assessment questionnaire in group A (treated with Meteospasmyl) exceeds the chances of an outcome in comparison with group B (other drugs) 22.7-fold at 95% CI 8.9–58.4.The cluster analysis results for a total of 5 items of the self-assessment questionnaire (group A – 86.7%, group B – 22.7%, OR=22.7) were used to analyze the adequacy of the power of the study (Power test: comparison of proportion, with significant of level 5% for 2 side and 2.5% for 1 side). The results obtained were as follows: POWER OF EXACT TESTS: Fisher’s test: 89.45%, Mid-P test: 93.70%; power of chi-square test: continuity-corrected: 8,08%, not continuitycorrected: 96,18%. Thus, the power of the study is above 80%, i.e. sufficient for an objective assessment. Statistical analysis results of the hand dynamometer grip strength in kilograms are shown in succession. Figure 2 shows the results of the difference in hand dynamometer values between visits 2 and 3 minus visit 1 in Group A. Statistical analysis showed a significant increase in the right-hand grip strength by 0.4 kg at visit 2 and by 0.6 kg at visit 3 compared to visit 1. Difference in kg by visit: F-test ANOVA (2; 60) = 7.2; p=0.0015; Kruskal-Wallis test-H (2; 63) = 27.6161; p=0.00001. The applied multivariate analysis of variance¬ with hypothesis decomposition model and sigma parameterization allowed to identify a significant effect of Meteospasmyl administration on the increase of hand dynamometry values on day 28, p trend less than 0.001. At the same time, a significant decrease in the hand grip strength was detected in Group B at the second and third visits compared to the first visit: median at 25/75 quartiles at the 2nd visit –0.9 (–1.2–0.0), at the 3rd visit –0.9 (–1.3 – –0.1). Difference in kg by visit: F-test ANOVA (2; 24) = 6.4; p=0.0059; Kruskal-Wallis test-H (2; 27) = 10.8; p=0.0045.
Safety Assessment
No clinical signs of adverse reactions were reported in both Groups. Table 5 shows the results of the descriptive statistics of laboratory and biochemical data in Group A for each visit individually and for all visits in aggregate. Visit comparison shows no significant difference (p trend over 0.05) in total protein in blood, total and conjugated (direct) bilirubin¬, creatinine, urea, glucose, cholesterol, hsCRP and AST, ALT, ALP enzyme activity. It should be noted that no mean and median values exceeding the upper reference values were detected for any of the indicators. However, in terms of upper 95% CI and/or 75th quartile of creatinine, glucose, amylase, cholesterol and ALT have values exceeding the upper reference values. To clarify the possible relationship with the treatment, it was necessary to assess the incidence of increased values at the 2nd and 3rd visits compared to the 1st visit. Creatinine above 80 μmol/L was observed in 5 subjects (23.8% at 95% CI 8.2–47.2) of 21 participants in Group A at three visits – all males for whom the upper reference value is 110 μmol/L, this level was not exceeded. Glucose above 5.5 mmol/L was recorded at visit 1 in 7 (33.3, 95% CI 15.9–55.1) cases, at visit 2 – in 7 cases (same as at visit 1), at visit 3 in 2 cases (9.5%, 95% CI 1.2–30.4) of 21, but the statistical difference was not significant (Chi-square = 3.451, P=0.063).Amylase above 60 U/L was detected at three visits in 5 (23.8%, 95% CI 8.2–47.2) of the same patients. No increase in amylase was detected at the 2nd and 3rd visits compared to the 1st visit (before treatment).
Table 5. Results of the descriptive statistics of laboratory and biochemical data in Group A for each visit individually and for all visits in aggregate.
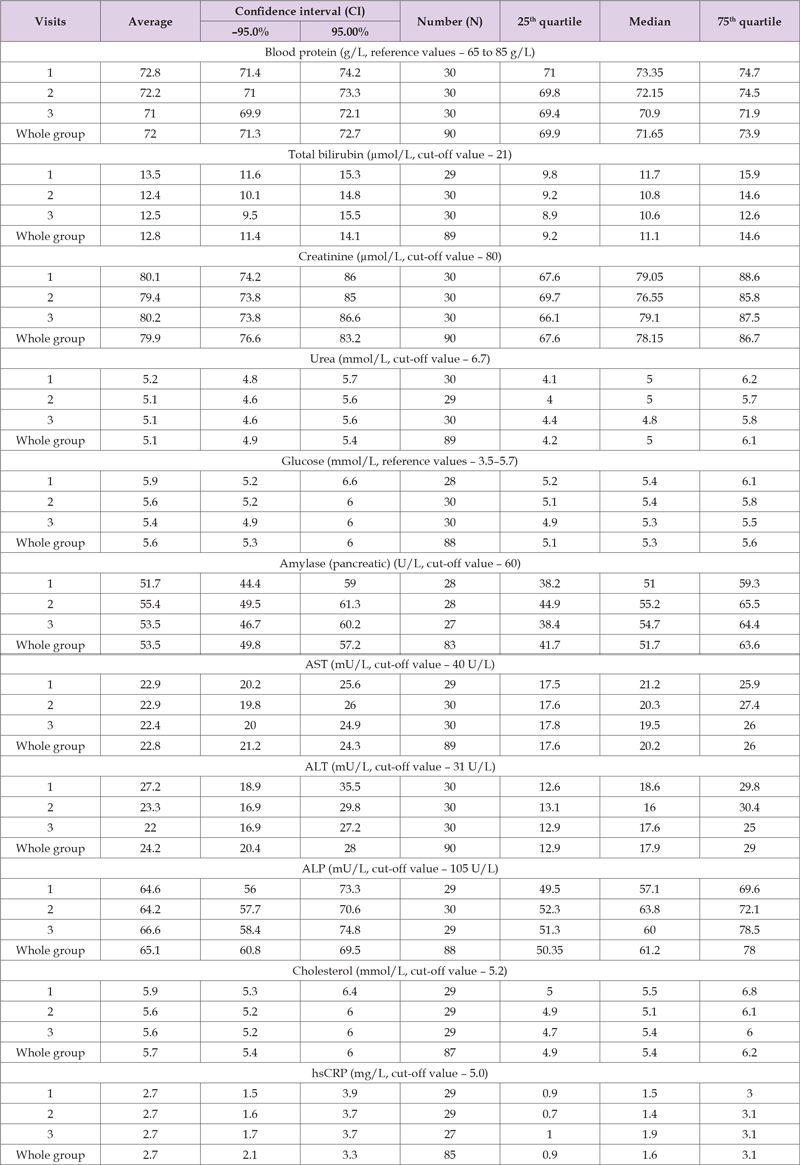
ALT above the upper reference value (31 U/L) was detected in 4 cases (19%, 95% CI 5.4–41.9) at three visits. At the same time, in all cases, the comparison of ALT values shows a decrease in values at the 2nd and 3rd visits compared to the first examination. No increase in ALT values by more than 10% (method error) from the level at the first visit was detected. Increased cholesterol values (above 5.5 mmol/L) were recorded in 6 patients (28.6%, 95% CI 11.3–52.2) at all three visits, with no significant increase at the 2nd and 3rd visits compared to the 1st visit. The above shows¬ the absence of increased laboratory and biochemical indicators during alverine with simethicone (Meteospasmyl) treatment compared to the pre-treatment period. Table 6 shows the results of the descriptive statistics of laboratory and biochemical data in Group B for each visit individually and for all visits in aggregate. In the comparison group (alternative treatment), comparisons between visits were made¬ by median and quartiles, taking into account the non-normal distribution and the number of observations. Visit comparison shows no significant difference (p trend over 0.05) in total protein in blood, total and conjugated (direct) bilirubin, creatinine, urea, glucose, cholesterol, hsCRP and AST, ALT, ALP enzyme activity. It should be noted¬ that no median values exceeding the upper reference values were detected for any of the indicators. However, some upper 75th quartile values of creatinine, glucose, amylase and cholesterol exceeded upper acceptable limits. To clarify the -possible relationship with the treatment, the incidence of increased values was assessed at the 2nd and 3rd visits compared to the 1st visit. Increased creatinine values were detected in 4 subjects (44.4%, 95% CI 13.7–78.8) and one subject had creatinine increase above 10 μmol/L after the start of treatment at the 2nd and 3rd visits compared to the 1st visit.
Table 6. Results of the descriptive statistics of laboratory and biochemical data in Group B for each visit individually and for all visits in aggregate.
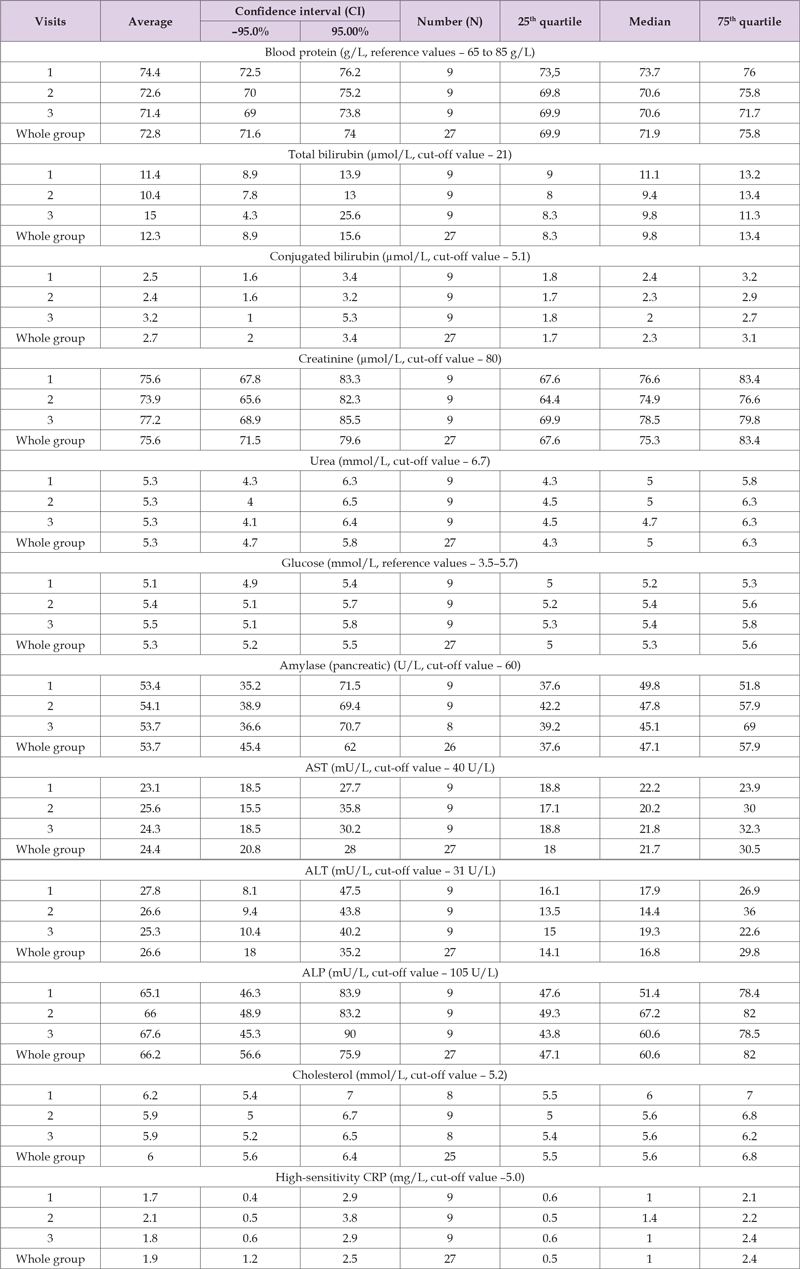
Thus, there was ¬an adverse reaction (11.1%, 95% CI 0.3–48.2). Blood glucose was increased (more than 5.5 mmol/L) in 5 patients (55.6%, 95% CI 21.2–86.3). At the same time, in all cases, increased values were recorded at the 2nd and 3rd visits, i.e. in the course of the treatment: in 3 ¬cases not more than 0.5 mmol/L, in 2 cases – more than 1.0 mmol/L. Thus, there was an adverse reaction in the form of mild hyperglycemia in 2 cases (22.2%, 95% CI 0.3–60.0). Increased amylase levels were observed in 2 cases (22.2%, 95% CI 0.3–60.0), at all three visits, with no increase at the 2nd and 3rd visits compared to the 1st visit. Cholesterol above 5.5 mmol/L was detected in 5 patients (55.6%, 95% CI 21.2–86.3), with no increase at the 2nd and 3rd visits compared to the 1st visit. The total number of adverse reactions for laboratory and biochemical ¬indicators was 3 (33.3, 95% CI 0.7-70.1). Comparison of adverse reactions by four-field table method (2×2 Table) according to laboratory and biochemical blood indicators of Groups A and B showed¬ significantly higher chance of their occurrence (OR-10.9) in comparison group (Group B) compared to main group (Group A): Chi-square (df=1) = 4.45, p=0.03; V-square (df=1) 4.30, p=0.04; PETO ODDS RATIO (A:B) = 10.9 [reciprocal = 0.09], Confidence intervals: 95% = 1.14–104.44 (Figure 2).
Table 7. Shows the results of the descriptive statistics of hematological data in Group A for each visit individually and for all visits in aggregate.
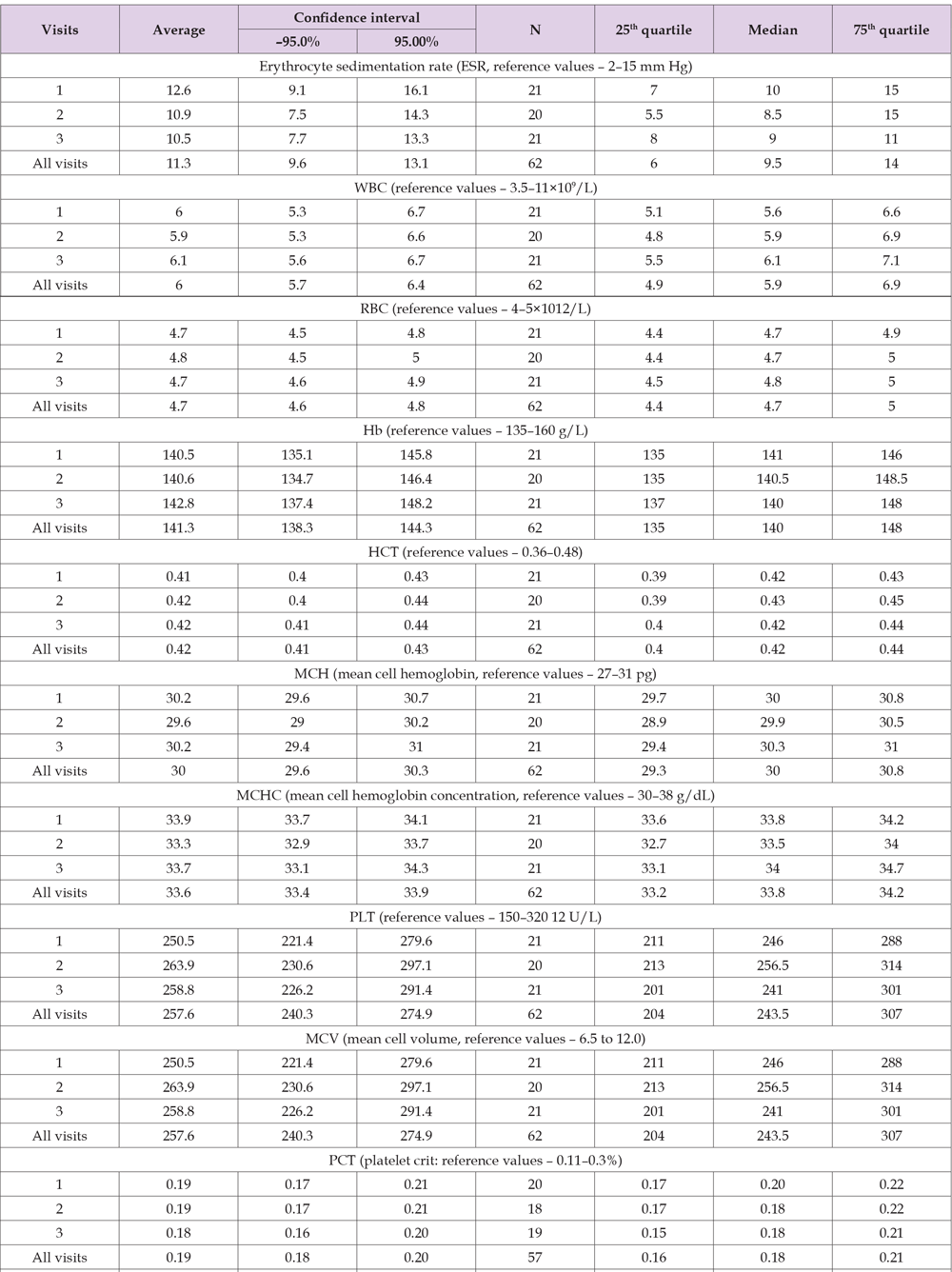
Analysis of Hematological Indicators
Based on the results presented in Table 7, hematological values at all visits were within reference limits for mean, median¬ and variance at 95% CI and 25-75th quartiles. No significant changes were detected between treatment visits compared to the first (no treatment) visit. No adverse reactions in terms of hematological indicators were detected during Meteospasmyl treatment.
Common Urine Analysis Indicators
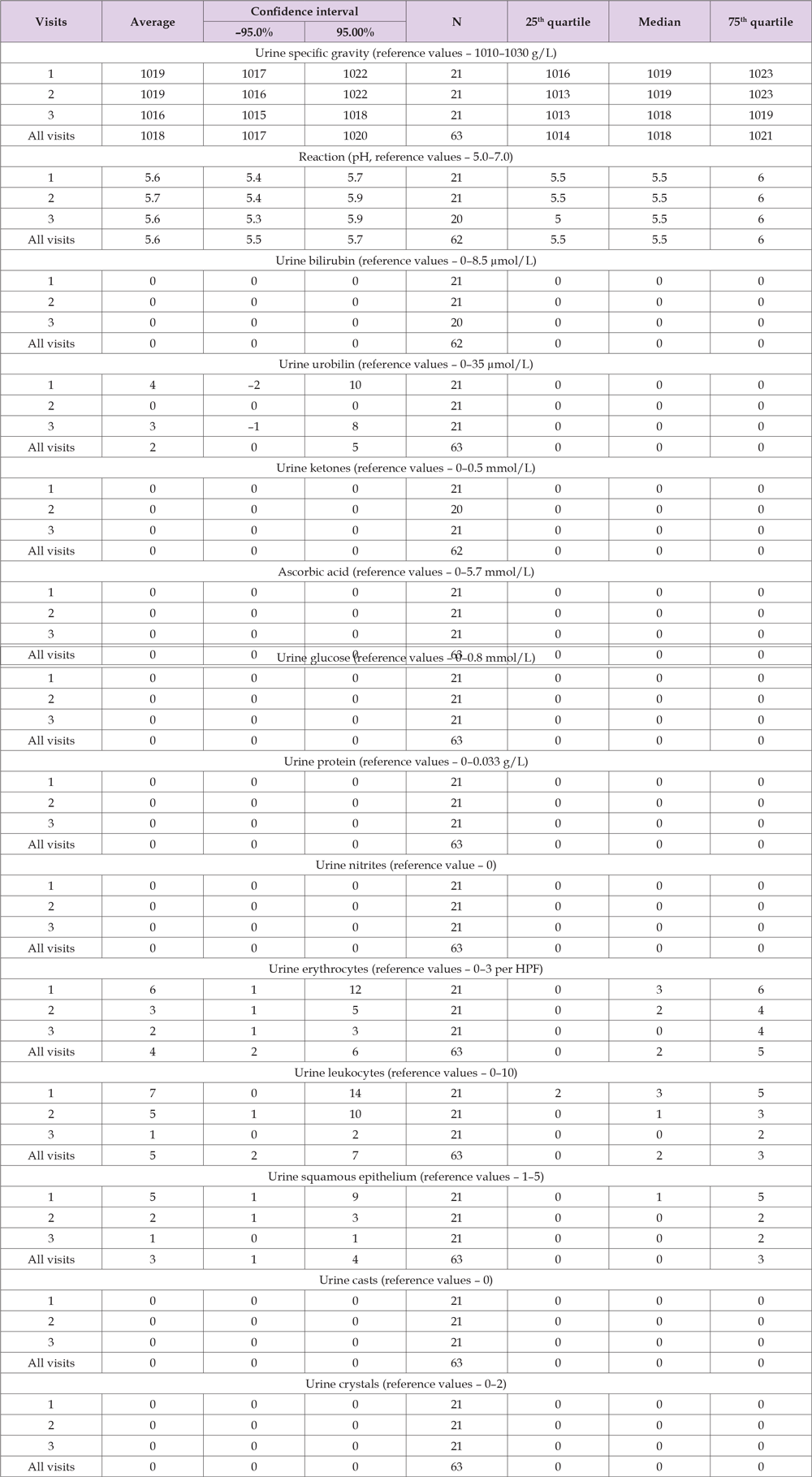
Table 8 shows the results of the descriptive statistics of urine analysis data in Group A for each visit individually and for all visits in aggregate. Based on the results presented in Table 8, urine analysis values at all visits were within reference limits for mean, median and variance at 95% CI, 25–75th quartiles. No significant changes were detected in the following urine analysis indicators: bilirubin, urobilirubinogen, ketones, ascorbic acid, glucose, protein, reaction (pH), nitrite, RBC, WBC, epithelium, casts, crystals. However, there are significant changes in the mean urine specific gravity values at the 3rd visit compared to the 1st visit, but they are statistically insignificant by the median. No other changes were detected between treatment visits compared to the first examination (no treatment). There were no adverse reactions to Meteospasmyl treatment in terms of urine analysis indicators.
Table 8. Results of the descriptive statistics of urine analysis data in Group A for each visit individually and for all visits in aggregate.
Conditions previously referred to as functional gastrointestinal disorders (FGIDs) have been changed to disorders of gut-brain interaction (DGBI) and represent multiple clusters of chronic gastrointestinal symptoms that occur in the absence of disease with topical and morphological (organic) changes [15,16]. A particular part of these clusters is represented by conditions with overlap syndrome. In total, there are about 33 DGBIs that can occur in any area of the gastrointestinal tract, IBS and FD are the most frequently recognized and studied of them. Practitioners have particular difficulties in overlapping symptoms, both in terms of differential diagnosis and treatment of such conditions. The situation is complicated by the complexity of putative pathophysiological mechanisms of DGBI formation [17] in the form of combinations of visceral hypersensitivity, dysmotility, transformed mucosa with disorders of its immune function, intestinal microbiota features and changes in the interaction between the central nervous system and the enteric nervous system (Top-down model and Bottom-up model) [18]. However, even for well-differentiated IBS forms, there are still no satisfactory treatment methods for patients due to its complex pathogenesis [19,20]. Currently, the emphasis is placed on symptomatic treatment with the use of various drugs that have little or no effect on the underlying complex multicomponent disease process, with about a third of patients failing to achieve the expected therapy efficacy [21]. The practical situation becomes even more complicated when overlapping symptoms. It should be noted that publications note significant differences in the reported incidence of intersection syndromes in DGBI and virtually no evaluation of the drug treatment efficacy and safety for such conditions [22,23]. It has also been shown that the natural history in people with IBS overlapping FD is more severe than in those with isolated IBS [24].
We were unable to find any publications on the subject of drug treatment of UD overlapping with nonspecific IBS-N form. Thus, all of the above demonstrates the need to conduct a clinical trial of the selected option of overlapping UD symptoms with IBS-N. The selected drug Meteospasmyl is a combination in one form of two active substances, alverine and simeticone. Alverine is a myotropic antispasmodic whose action is not accompanied by atropine-like effect or ganglion blocking activity. Simethicone properties are sufficiently well-known to practitioners, which is not the case with alverine. Besides, according to the Anatomical Therapeutic Chemical Classification System (ATC), simethicone belongs to the group of silicones – A03AX13, alverine belongs to the same group – A03AX08, i.e. to A03AX – Other drugs for functional gastrointestinal disorders (WHO ATC DDD Index: https://www.whocc.no/ atc_ddd_index/?code =A03AX&showdescription=no).
It has been shown that alverine can enhance calcium (Ca) influx during action potentials by inhibiting the inactivation of type l calcium channels but can also inhibit evoked activity by inhibiting the sensitivity¬ of contractile proteins to Ca2+. The proportional contribution of Ca-dependent and Ca-independent contractions in the monofascicular detrusor smooth muscle (DSM) stretch can vary between spontaneous and evoked activity, which requires further trials of the interactions between these pathways to assess the therapeutic potential of alverine for DSM dysfunction treatment [25]. In terms of chemical and pharmaceutical properties, alverine is N-Etyl-3,3’- diphenyldipropylamine, used as Alverine Citrate.A PubMed database search for publications on alverine and/or alverine with simethicone found 85 publications in the past 10 years. According to available publications, alverine is a smooth muscle relaxant (antispasmodic) used for the relief of stomach convulsions and enterospasms accompanied by pain syndrome. Anti-inflammatory action of alverine has been additionally reported in vitro and in vivo. Thus, the 2020 publication [26] shows the following: the production of nitric oxide (NO) in RAW264.7 cells, activated by lipopolysaccharide (LPS) or polyinosine (polycytidylic acid (poly(I:C)), decreased under the action of alverine. The expression of mRNA-induced nitric oxide synthetase (iNOS), cyclooxygenase-2 (COX-2) and tumor necrosis factor alpha (TNF-α) was also dose-dependently inhibited by alverine treatment. In reporter gene assays, alverin clearly reduced luciferase activity mediated by nuclear factor transcription factor κB (NF- κB) in HEK293 cells containing the TIR domain, adapter-inducing interferon-β (TRIF) or MyD88 with overexpression. In addition, phosphorylation of NF-κB subunits and upstream signaling molecules including p65, p50, AKT, IκBα and Src was inhibited by 200 μM alverin in LPS-treated RAW264.7 cells. Using immunoblotting and cellular thermal shift assay (CETSA), Src was identified as a target of alverin in its anti-inflammatory response. In addition, alverine at doses of 100 and 200 mg/kg is effective in HCl/EtOH-induced gastric ulcer in mice. Alverine reduces inflammatory responses by acting on Src in the NF- κB pathway, and these results justify the anti-inflammatory effect of alverine. Alverine is mentioned (p. 131, 156) in a discussion of the treatment of irritable bowel syndrome in a recent 2020 review [27]. A trial was conducted in Mexico to clarify the efficacy, safety and effect of alverine/simethicone (Meteospasmyl) in the treatment of patients with irritable bowel syndrome [28]. The authors used decision analysis to calculate the cost-effectiveness of three competing IBS treatment strategies:
1. Alverine/simethicone (Meteospasmyl).
2. Pinaverium bromide (PB).
3. Tegaserod (T).
A decision tree was built for a time horizon of 1 month, and then a statistical Markov model was built for 13 months-this model was implemented in two scenarios. The first Markov model studied the treatment of a patient with only one drug therapy, while the second analyzed diagnostic findings of a patient who was treated with a change in treatment if he or she did not respond to the first option. Overall symptom reduction and duration (time) without symptoms were estimated. Additionally, direct treatment costs were estimated using an incremental cost-effectiveness ratio (ICER) and sensitivity and likelihood analyses were performed. The authors found that Meteospasmyl was more effective and less costly in the treatment of IBS; in a Markov model, Meteospasmyl compared to PB was the dominant strategy. Sensitivity analysis showed that Meteospasmyl was more cost-effective than PB and T in treating patients with IBS in Mexico. According to the probabilistic sensitivity analysis, the probability of Meteospasmyl’s profitability was 90% below the threshold of willingness to pay for the drug in Mexico. The authors concluded that the results of the clinical trial and cost-effectiveness evaluation showed that the use of Meteospasmyl in the treatment of patients with IBS was cost-effective and should be considered as the first treatment option for patients diagnosed with IBS.
In our trial, the high efficacy of Meteospasmyl according to patient self-assessment was noted, with a positive effect rate reaching 86.7% (95% CI 78.6-92.5), characterized by a high chance (22.7, 95% CI 8.9-58.4) of achieving an effect compared to the comparison group (treatment without Meteospasmyl). Thus, Meteospasmyl can be used as a first-line treatment for overlapping dyspepsia with a nonspecific irritable bowel syndrome, especially while waiting for a definitive examination of patients. This statement should be supplemented with results showing a high degree of safety of this medicinal product in both clinical and laboratory parameters. In 2021, a review by American authors on the efficacy and safety of antispasmodics available in North America (alverine, dicyclomine, hyoscine, hyoscyamine, mebeverine, oticonium, pinaverium and trimebutine) for the treatment of chronic abdominal pain was published [29]. Overall on antispasmodics, the authors noted that there is limited evidence to support the use of antispasmodics for the treatment of chronic abdominal pain in patients with gut-brain interaction disorders, including irritable bowel syndrome, functional dyspepsia and centrally mediated abdominal pain syndrome. The small sampling size, short-term therapy, heterogeneity of results and concerns about potential systematic error in trial design make it difficult to recommend these drugs for clinical use, especially when comparing them to the data sets available from large randomized controlled trials that characterize current FDA-approved drugs for IBS therapy. In particular, this publication on alverine presents the following data: efficacy and safety of alverine, calcium channel blocker, was studied in 2 randomized placebo-controlled trials. A comparable percentage of patients receiving alverine at a dose of 120 mg t.i.d. or placebo for 12 weeks showed improvement from baseline in the intensity and frequency of abdominal pain, abdominal bloating and general well-being at week 12, but the differences between groups did not reach statistical significance. Fewer patients receiving alverine reported adverse reactions (≥1 HF) compared to those receiving placebo. In the second trial, alverine 60 mg/simethicone 300 mg t.i.d. was significantly more effective than placebo in reducing abdominal pain in patients with IBS (P=0.047). The safety profile of alverine/ simethicone was generally comparable to that of placebo, but this trial potentially excluded patients with more severe symptoms.
Attention should be drawn to a publication by the Korean authors [30]. The authors evaluated the effect of alverine citrate (AC) on sarcopenia, the presence of which significantly alters bowel motility. The authors point out the following. Currently, there are no pharmacological drugs available for beneficial sarcopenia treatment. This trial focused on the screening of a drug library using the atrogin-1/MAFbx promoter assay, which identified candidate drugs capable of reducing muscle atrophy. The selected candidate drug was also investigated for its use in the treatment of sarcopenia by evaluating its efficacy in vitro and in vivo. The authors determined the daily dose of AC (73.8 mg/kg) in mice, which corresponds to the recommended human dose used for antispasmodic therapy, in 120 mg capsules t.i.d. AC administration at this dose (73.8 mg/kg) to mice clearly increased muscle mass and improved physical capabilities. It has been demonstrated that AC reduced muscle weakness caused by age and immobilization. The authors of this publication believe that the¬ findings represent a new strategy for safe therapeutic intervention in the treatment of muscle diseases accompanied by sarcopenia.
The presented study corresponds to one of the current areas of research in the European region, in accordance with the one noted in the latest publication (White Book 2) for investigation of research gaps and priorities in the field of digestive health in the European Region [31]. The findings obtained in our study confirm the effect of Meteospasmyl on the skeletal muscles with a significant increase in right¬ hand grip strength by 0.4 kg at the 2nd visit and by 0.6 kg at the 3rd visit compared to the 1st visit (F-test ANOVA (2; 60) = 7,2; p=0.0015; Kruskal – Wallis test-H (2; 63) = 27.6161; p=0.00001). However, the applied multivariate analysis of variance with hypothesis decomposition model confirmed such effect of Meteospasmyl on the increase of hand dynamometry values on day 28 (p trend less than 0.001). The advantages of the presented study are the following:
1. New data were obtained for the option of overlapping
uninvestigated dyspepsia with nonspecific irritable bowel
syndrome.
2. Patients’ self-assessment of efficacy in this option has not
been studied before.
3. Meteospasmyl improved functional status of skeletal
muscles;
4. Meteospasmyl showed a high safety profile.
Marakhouski Y.(ORCID 0000-0001-7327-7762) – general management of the study; analysis of the available preliminary results of studies on the syndrome of overlap in functional diseases of the digestive system, in order to control the conditions of the study and possible changes in the study plan, with the development of an adequate study design; identification and formulation of the conflict of current statements and results, in comparison with those assumed in this study, with an assessment of their novelty; development of an original research plan for the most effective testing of a new hypothesis; evaluation of the fidelity of the preliminary hypothesis, new facts and prospects for further research; the choice of adequate statistical analysis and models for comparing results to level confounding effects and increase the validity of the study results; there is no conflict of interest; Zharskaya O.( 0000-0002-3511- 0707) – interpretation of the results of statistical analysis; graphical and numerical representation of the results of statistical analysis; comparison of the obtained results with those published earlier; identification and formulation of the conflict of current statements; Karasiova G. – evaluation of the discussion section in terms of clarifying contradictions and conflicting statements.
Nothing to declare.


