Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Wang Zhengfeng1, Dong Shi1, He Ru1 and Zhou Wence2*
Received: March 13, 2023; Published: March 27, 2023
*Corresponding author: Zhou Wence, The Second Hospital of Lanzhou University, Lanzhou, Gansu 730000, China
DOI: 10.26717/BJSTR.2023.49.007800
When pancreatic tissue is damaged by external force or inflammation, it initiates its own repair process. Like most tissue damage repair, the repair process of pancreatic injury also includes the repair of cellular and interstitial components. The repair of cell components is achieved through cell division and proliferation, while the repair of interstitial components is mainly completed through the secretion of extracellular matrix (ECM). As the main cellular component of pancreatic tissue, pancreatic stellate cells (PSCs) participate in the secretion of ECM and the differentiation and proliferation of other pancreatic parenchymal cells through various pathways. A large number of studies have confirmed that the activation of PSC plays an important role in the repair of pancreatic injury. Based on a large number of literature study, this paper summarizes the current research progress on the mechanism of PSC in the repair of pancreatic injury, in order to provide new directions and ideas for the basic research and clinical diagnosis and treatment of pancreatic injury.
Keywords: Pancreatic; PSC; ECM; Damage Repair; Signaling Pathway
Pancreatic Stellate Cell
PSC, which is stellate or fusiform, is relatively rare, accounting for only 4%-7% of the total number of pancreatic cells. In 1982, Japanese scholars [1] found a kind of cells with vitamin A storage function similar to hepatic stellate cells in mouse pancreas. In 1998, (Apte, et al. [2]) isolated rat resting PSC by gradient centrifugation, and (Bachem, et al. [3]) isolated active PSC by tissue block culture. In healthy pancreatic tissues, PSC is in a resting state. When stimulated by various injury factors, PSC is activated and participate in the repair of damaged pancreas [4,5], cell volume increases, cell proliferation is active, and cells synthesize a variety of ECM components such as collagen, fibronectin and laminin. And produce a large number of cytokines (such as IL-1, IL-6, TNF-α, etc.), transforming growth factor β1(TGF-β1), chemokines and adhesion molecules, which promote the chemotaxis, aggregation and adhesion of inflammatory cells. And further promotes the secretion of ECM, accelerate wound healing [6-10]. Based on a large number of literature study, this paper summarizes the current research progress on the mechanism of PSC in the repair of pancreatic injury, in order to provide new directions and ideas for the basic research and clinical diagnosis and treatment of pancreatic injury.
Interacts With Other Cells of the Pancreas
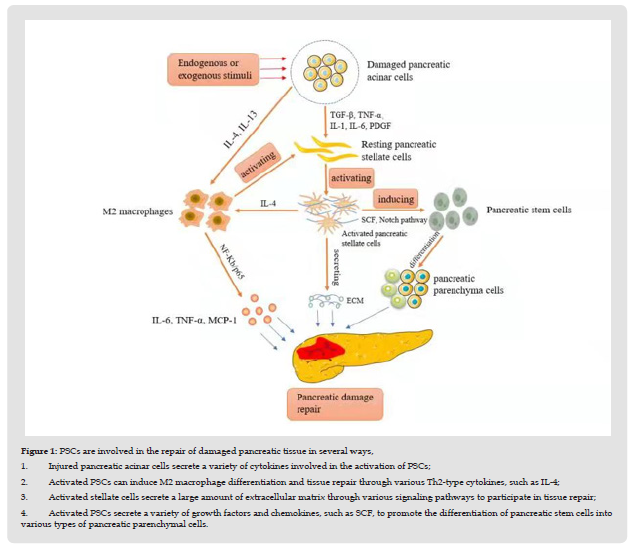
Pancreatic acinar cells, macrophages, PSCs and stem cells mainly exist in the microenvironment of pancreatic tissue, and they interact and participate in the fibrosis process of pancreatitis tissue [11,12]. Studies have found [13] that fibroblasts in pancreatic ductal adenocarcinoma can interact with stem cells, acinar cells and macrophages, regulate the functions of these cells, and promote the proliferation and differentiation of tumor cells and stromal cells. It is speculated that PSCs may play a similar role in the repair of damaged pancreas (Figure 1). to participate in tissue repair; (4). Activated PSCs secrete a variety of growth factors and chemokines, such as SCF, to promote the differentiation of pancreatic stem cells into various types of pancreatic parenchymal cells.
Figure 1 PSCs are involved in the repair of damaged pancreatic tissue in several ways, 1. Injured pancreatic acinar cells secrete a variety of cytokines involved in the activation of PSCs; 2. Activated PSCs can induce M2 macrophage differentiation and tissue repair through various Th2-type cytokines, such as IL-4; 3. Activated stellate cells secrete a large amount of extracellular matrix through various signaling pathways to participate in tissue repair; 4. Activated PSCs secrete a variety of growth factors and chemokines, such as SCF, to promote the differentiation of pancreatic stem cells into various types of pancreatic parenchymal cells.
Regulates Differentiation of Pancreatic Stem Cells into Parenchymal Cells
The proliferation of pancreatic parenchymal cells plays a very important role in the repair of damaged pancreas. Several studies have found [14,15] that pancreatic stem cells exist in pancreatic acinar and islet tissue and can be induced to differentiate into pancreatic endocrine cells, ductal cells and exocrine cells. Some scholars [16] injected 5-ethynyl-2’deoxyuridine-labeled stem cells and granulocyte colony-stimulating factor into a rat model and found that stem cells distributed in pancreatic tissue not only antagonize the release of inflammatory mediators, reduce the immune response and other aspects control the acute inflammatory response, and it can also differentiate into pancreatic acinar cells and endothelial cells to repair the damaged pancreas. PSCs play an important role in promoting the proliferation and differentiation of stem cells and MSCs. First, PSCs activated after pancreatic tissue damage can secrete a variety of growth factors and chemokines, which play a role in the differentiation of stem cells into pancreatic parenchyma cells. to positive promotion. [17] detected significantly increased SCF mRNA and protein expression in activated rat PSCs by RT-PCR technology, and SCF played a huge role in the differentiation and growth of rat stem cells. Second, previous studies [7,18] found that some PSCs isolated from rat pancreatic tissue expressed a variety of stem/progenitor cell markers, such as CD133 and PITX2. In addition, as signaling pathways necessary for stem cell maintenance and development, β-catenin-dependent Wnt and Notch signaling are also involved in the cellular regulation of PSCs, suggesting that PSCs have stem cell-specific expression profiles with long-term survival, transplant ability and regenerative capabilities. Although the source of pancreatic stem cells is still controversial, according to the above research and analysis, the function of PSC is not only limited to the secretion of ECM, but also can induce the differentiation of stem cells into pancreatic parenchyma cells. At the same time, PSC is also a kind of cells with differentiation potential. by differentiating into parenchymal cells with endocrine and exocrine functions, and then replenishing and repairing pancreatic necrotic tissue from the cellular level when the pancreas is injured.
Pancreatic Acinar Cells Induce PSC Activation
Pancreatic acinar cells are mainly manifested as necrosis and apoptosis in pancreatic injury [19]. In the early stage of pancreatic injury, pancreatic acinar cells are damaged and induce inflammatory cell infiltration, which secretes TGF-β, tumor necrosis factor-α (TNF-α), IL-1, IL-6, platelet-derived growth factor (PDGF) and other inflammatory cytokines induce PSC activation [7,20]. In addition, studies have found [21] that, in addition to cell necrosis and apoptosis, autophagy dysfunction of pancreatic acinar cells is also an important factor that induces PSC activation. Injury of autophagic flow can activate the p62-TRAF6-NF-κB pathway, induce pancreatic acinar cells to release a large amount of cytokines, and promote PSC activation, resulting in the massive secretion of extracellular matrix and the differentiation and proliferation of pancreatic parenchyma cells. (Masamune, et al. [22,23]) demonstrated that pancreatic cancer cell-derived exosomes can activate extracellular signal-regulated kinase (ERK) and serine/threonine kinase (Akt) in PSCs. And increase the mRNA expression of its α-SMA and fibrosis-related genes and promote the production of type I procollagen C-peptide, thereby activating PSCs. However, whether damaged pancreatic acinar cells can also promote PSC activation through their derived exosomes to promote ECM secretion remains to be further investigated.
Interact with Macrophages
Macrophages can differentiate into different phenotypes due to different microenvironments in vivo, which are called macrophage polarization, mainly including two types, M1 and M2. Among them, M1 type mainly exhibits pro-inflammatory effect, and M2 type is a selective activated macrophage, which has anti-inflammatory and pro-fibrotic effects, and can be activated by Th2-type cytokines such as IL-4 and IL-13 [24]. (Sendler, et al. [25]) stimulated acinar cells with cholecystokinin to cause their damage and then co-cultured with macrophages, and found that the damaged acinar cells could promote the differentiation of macrophages into M2 type due to the release of chemokines and inflammatory factors, thus secreting a large number of pro-inflammatory cytokines such as IL-6, TNF-α and chemokine protein-1. Participates in the repair of pancreatic injury. In the early stage of pancreatic injury, M2-type macrophages can activate PSCs, causing the secretion of pancreatic ECM [26,27]. In the middle and late stages of pancreatic injury, activated PSCs can also produce Th2-type cytokines such as IL-4, induce macrophages to differentiate into M2-type cells, further promote the secretion of ECM in pancreatic tissue, and accelerate injury healing [27]. (Michalski, et al. [28]) established a co-culture system of macrophages and PSCs, and found that macrophages can not only promote PSC activation, but also enhance the autocrine capacity of PSCs, which may lead to a persistent chronic inflammatory state.
Enhanced EMT Pathway and Promotes Injury Healing
Epithelial-mesenchymal transition (EMT) refers to the biological process in which epithelial cells are transformed into cells with a mesenchymal phenotype through specific procedures. In addition to causing changes in cell surface proteins and cytoskeleton, it also leads to changes in the production of ECM proteins. It plays an important role in embryonic development, chronic inflammation, tissue remodeling and various fibrotic diseases. There are 3 types of EMT, of which type 2 EMT converts epithelial cells into fibroblasts and is closely related to the secretion of ECM in tissues [29]. Recent studies [30] believe that the activation process of PSC has very similar morphological and functional changes to the process of EMT. The expressions of markers (N-cadherin, vimentin, fbronectin1 and S100A4) were up-regulated, and EMT-related transcription factors were also up-regulated, all of which were consistent with the characteristics of EMT. Therefore, it is believed that PSC can play an important role in the repair of damaged pancreatic tissue through EMT, and its activation is actually a change from a resting state to a fibroblast-like change, which is an EMT-like process.
PSCS Regulate Profibrotic Effects Through Autophagy
Autophagy is a process that relies on the lysosomal pathway to degrade cytoplasmic proteins and organelles. Studies have found that in an animal model of chronic pancreatitis, the application of the PI3K/Akt/mTOR pathway inhibitor LY294002 to PSCs cultured in vitro can inhibit the PI3K/Akt/mTOR signaling pathway in PSCs and enhance the level of autophagy in pancreatic tissue [31-33]. Downregulation of retinoblastoma coiled coil protein1 (RB1CC1) expression level in mouse models inhibits PSC autophagy level and alleviates pancreatic fibrosis. Similarly, it has been found in patients with chronic pancreatitis. RB1CC1 expression level is positively correlated with the degree of pancreatic fibrosis, and it promotes PSC activation through its interaction with ULK1 [34]. Therefore, it is speculated that PSCs may degrade intracytoplasmic lipid droplets through autophagy, providing raw materials and energy for the activation of quiescent PSCs, thereby promoting the pro-ECM secretion of PSCs and accelerating the repair of damaged pancreas.
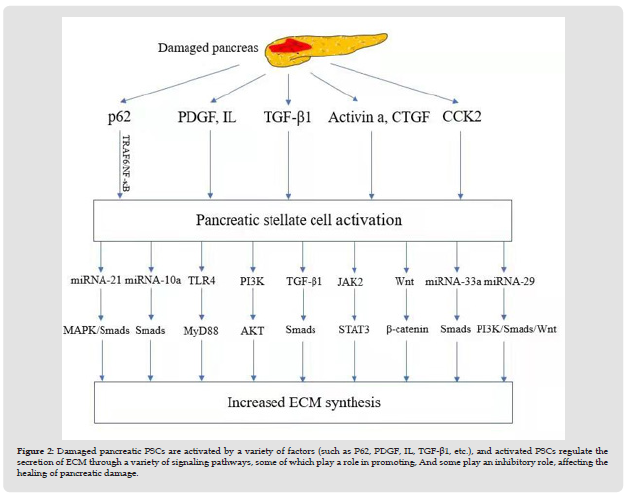
Figure 2 Damaged pancreatic PSCs are activated by a variety of factors (such as P62, PDGF, IL, TGF-β1, etc.), and activated PSCs regulate the secretion of ECM through a variety of signaling pathways, some of which play a role in promoting, And some play an inhibitory role, affecting the healing of pancreatic damage.
Signaling Pathways Regulating PSC Extracellular Matrix Secretion
After pancreatic injury, PSCs are activated by a variety of cytokines, such as TGF-β1, CTGF, PDGF, IL, activin A, Galectin-1 and oxygen free radicals. Finally, it regulates the pro-fibrotic process of PSC by activating intracellular signaling pathways such as TGF-β/ Smads, JAK2/STAT3, PI3K/AKT, Wnt/β-catenin and TLR4 [35]. Activated PSCs can also induce more PSC activation through autocrine secretion of inflammatory factors such as CTGF, TGF-β1, and PDGF. Activated PSCs have the ability to proliferate, migrate, synthesize ECM, and accelerate the secretion of pancreatic extracellular matrix [7] (Figure 2).
TGF-β1/Smads Signaling Pathway
Previous studies have found that PSCs can autocrine TGF-β, and TGF-β, as an important regulator of fibrotic disease, can regulate the transformation of fibroblasts to myofibroblasts, and further induce PSC activation and FN synthesis [36-38]. Among the various subtypes, TGF-β1 has the highest expression level and the strongest activity in all tissues and is widely involved in the occurrence and development of tissue fibrosis and tumor and other physiological and pathological processes [39-41]. Several studies have shown that the activation of PSC and the expression of TGF-β1 mRNA are related to the Smad2/3 signaling pathway [42], and TGF-β1 can participate in the repair process through the classic TGF-β1/Smads signaling pathway in injured pancreatic tissue, can be involved in pancreatic wound healing, cell growth, cell cycle regulation, angiogenesis, and immune regulation [43,44].
JAK2 /STAT3 Signaling Pathway
The Janus kinase /signal transducer and activator of transcription3 (JAK /STAT3) signaling pathway is the way that most cytokines transmit information into cells, thereby inducing the expression of target genes and participating in cell proliferation, differentiation, apoptosis and immune regulation and many other important biological processes. (Komar, et al. [45]) Found that in a mouse model of cerulein-induced chronic pancreatitis, administration of a Jak1/2- STAT3 phosphorylation inhibitor for one week could significantly reduce the inflammatory response and biomarkers of fibrosis in damaged pancreatic tissue substances that inhibit PSC activation. In PSC activated by TGF-β1, JAK2 /STAT3 signaling pathway was activated, α-SMA expression was increased, and the secretion levels of inflammatory cytokines IL-6 and IL-1β were also increased. While after adding JAK1/2 inhibitor or STAT3 interfering RNA, The JAK2 / STAT3 signaling pathway is inhibited, the activation of PSC is reduced, and the level of secreted inflammatory cytokines is downregulated [45]. All these evidence indicate that the Jak/STAT pathway plays a huge role in the activation and proliferation of PSCs.
PI3K /AKT Signaling Pathway
The PI3K/AKT signaling pathway is an important signaling pathway in cells, which plays a role in rapid signal transduction from the cell membrane to the nucleus. This signaling pathway can be activated in various ways and is involved in cell proliferation, differentiation, migration, and apoptosis. (Xu, et al. [46]) found that TGF-β1 upregulates PTEN expression, inhibits the activation of PI3K/AKT/mTOR signaling pathway, and promotes autophagy in PSCs, thereby inducing PSC activation and ECM secretion. (Xue R, et al. [47,48]) treated PSCs isolated from mice with different doses of CoQ10 and found that compared with the control group, the expression of desmin, p-PI3K, p-AKT and p-mTOR were significantly increased in PSCs in the CoQ10-treated group, but at the same time, the levels of collagen I and collagen III proteins secreted by PSCs were significantly decreased, suggesting that CoQ10 inhibits the activation of PSCs by activating the PI3K/AKT/mTOR signaling pathway, thereby reducing the secretion of ECM. However, some studies [49] found that a natural flavanol glycoside (EruberinA) inhibited the PI3K /AKT signaling pathway while inhibiting the expression levels of α-SMA, FN and collagen I in pancreatic rat PSCs activated by TGF-β. These results suggest that PI3K /AKT signaling pathway may play a role in promoting ECM secretion by inducing PSC activation. Although the PI3K/AKT signaling pathway is currently widely used in tumor-related research, existing studies have shown that it also plays an important regulatory role in pancreatic fibrosis, especially in promoting PSC secretion of ECM, but its specific regulatory direction and the mechanism remains to be further studied.
Wnt/β-Catenin Signaling Pathway
The Wnt signaling pathway is highly conserved in multicellular organisms and plays an important regulatory role in cell development and disease progression [50,51]. Several recent reports suggest that Wnt signaling plays an important role in the development of fibrosis in multiple organs [52,53]. (Yanling Hu, et al. [54]) studied the rat PSC cells induced by bombesin and found by qRT-PCR technology that compared with the PSC in the resting state, the genes Wnt1, 2, 3a, 10b and β-catenin were significantly overexpressed in the PSC cells which were cultured for one week in vitro. Furthermore, it was found by fluorescence immunoassay that Wnt2 and β-catenin were mainly located in the nucleus of PSCs and were significantly expressed. This illustrates the role of Wnt/β-catenin signaling pathway in the activation of PSCs.
TLR4 Signaling Pathway
Toll-like receptors (TLRs) are currently considered to be the only key transmembrane proteins in mammals that transmit extracellular antigen recognition information into cells and trigger inflammatory responses. Toll4 is a member of the TLRs family and is a kind of pattern recognition receptor. It can act through myeloid differentiation factor 88 (MyD88)-dependent and MyD88-independent signaling pathways. Studies have found [55] rat PSCs express a variety of TLRs (TLR2, 3, 4 and 5 and related molecules CD14 and MD2) and respond to TLR ligands, leading to activation of signaling pathways and proinflammatory responses. It is inferred from this that TLR4 may be one of the important pathways for PSC to play a role in the secretion of pancreatic ECM, but the specific mechanism needs to be further studied.
Others
In addition to the above signaling pathways, Hedgehog (Hh) signaling pathway, rho-Rock signaling pathway, peroxisome proliferator-activated receptor γ(PPAR-γ) signaling pathway are also important molecular mechanisms of pancreatic fibrosis. HGF, PDGF, CX3CR1 and interleukin have also been confirmed to be directly or indirectly involved in the fibrosis process of chronic pancreatitis [56- 60]. Moreover, the above-mentioned various signaling pathways and cytokines interact with each other. A large number of studies have found that PSC-derived exosomes contain a variety of microRNAs, which play an important role in mediating information transmission between cells, and also through a variety of signaling pathways regulate the process of PSC promoting ECM secretion, For example, microRNA-33a and microRNA-10a can trigger the secretion of ECM such as collagen through the Smads signaling pathway [61,62]; the miRNA-29 family can block the PI3K signaling pathway [63,64] and inhibit the Smads signaling pathway [65] or Wnt/β-catenin signaling pathway [66], which plays a negative regulatory role in the process of ECM secretion; microRNA-21 can activate the MAPK signaling pathway, inhibiting apoptosis and promoting proliferation of pancreatic fibroblasts [67]; the up-regulated microRNA-21 also plays a promoting role in the Smads signaling pathway activated by TGF-β1, resulting in the increase of ECM synthesis capacity [68].
In summary, we see that PSCs play an extremely important role in the process of pancreatic injury repair. First, it can regulate the differentiation of pancreatic stem cells to other parenchymal cells of the pancreas, and even transform into stem cells and progenitor cells with differentiation potential through changes in their own phenotypes; PSCs also interact with pancreatic acinar cells, macrophages and other parenchymal cells, On the one hand, it regulates the proliferation ability and cell function of the above cells; on the other hand, the above parenchymal cells that are stimulated by damage will also regulate the function of PSCs and promote the secretion of ECM; Second, activated PSCs modulate the pro-fibrotic effect of PSCs and further promote wound healing by enhancing their own EMT pathway and autophagy; Finally, under the action of various factors (or autocrine), PSC can promote the secretion of extracellular matrix around trauma through various signaling pathways, and jointly complete the repair of injured pancreatic tissue.
Although it is currently believed that the repair process of the damaged pancreas is closely related to the massive secretion of ECM, PSCs have been intensively studied as a core target of pancreatic injury due to their ability to generate ECM. However, the repair of pancreatic injury is an extremely complex process, which includes regeneration of parenchymal cells and proliferation of interstitial fibers. The author believes that the following aspects will be the focus of future research on pancreatic injury repair:
a) Pancreatic stem cells play an important role in the process of pancreatic parenchymal cell regeneration, and many studies have found that PSC plays an important role in the differentiation of stem cells. However, the specific pathway through which PSC activates stem cells and the specific mechanism of regulating PSC’s differentiation into differentiated stem cells remains unclear. Further basic research is needed to explore.
b) The role of several common cells in the pancreatic tissue microenvironment in the repair of pancreatic injury has received widespread attention, but the mechanism through which various cells in the pancreatic microenvironment produce effects is still unclear. With the regulation of the function and molecular level of stem cells, acinar cells, PSC and macrophages, As well as in-depth studies on the interaction between cytokines and inflammatory mediators, multi-target intervention is likely to become a research hotspot of pancreatic injury repair, as well as a key point for the prevention and treatment of pancreatic fibrosis.
c) Although we have a certain understanding of psC-related signaling pathways in pancreatic tissue repair, various signaling pathways and factors interact with each other and their mechanisms are complex, and PSC has a wide range of subsets of variation due to different cell surface markers and functional differences, and these subsets have functional heterogeneity. Therefore, it is of great importance to find common regulation points of various signaling pathways.
d) A large number of studies have found that PSC-derived exosomes play an important role in mediating the process of intercellular information transmission, regulating the process of PSC promoting ECM secretion through a variety of signaling pathways. Further research and exploration of microRNA in PSC-related exosomes may become a new direction of targeted research on PSC activation pathway.
e) At present, most of the above basic studies on pancreatic injury repair are based on acute and chronic pancreatitis. There are few reports on the role and mechanism of PSC in pancreatic repair after trauma or iatrogenic pancreatic injury, and further studies are needed.


