Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Md. Monirul Islam1, Firoz Ahmed1,2 and Md. Ibrahim H. Mondal1*
Received: February 16, 2023; Published: March 23, 2023
*Corresponding author: Md. Ibrahim H Mondal, Polymer and Textile Research Lab, Department of Applied Chemistry and Chemical Engineering, Rajshahi University, Rajshahi-6205, Bangladesh
DOI: 10.26717/BJSTR.2023.49.007795
Due to their enhanced antibacterial activity and minimal cytotoxicity, nanocomposite hydrogels have been considered attractive materials for biomedical applications. Maleic acid was used as a crosslinker in the development of methylcellulose/PVP/ZnO nanocomposite hydrogels. FESEM, FTIR, EDX, hemolytic and antioxidant tests were carried out to evaluate the prepared nanocomposites. FESEM and EDX measurements confirmed the presence of ZnO nanoparticles in the polymer matrix. The DLS revealed testing that the particle’s maximum diameter is 50.7 nm, confirming the particle size and stability of the biogenic ZnO NPs. ZnO NPs incorporated nanocomposites exhibited excellent biodegradability and enhanced antioxidant activity. MC/PVP/ZnO nanocomposites exhibited a significant zone of inhibition against E. coli and S. aureus bacteria. The results showed that the prepared nanocomposites have high porosity (>80%), swelling properties, and antioxidant activity (>35%). The hemolytic activity test demonstrates that the developed nanocomposites are nontoxic. These findings significantly support the use of these innovative MC/PVP/ZnO bio-nanocomposite dressings for wound healing applications.
Keywords: Nanocomposite; Methylcellulose; Zinc Oxide Nanoparticles; Wound Healing
Chronic wounds are still incredibly difficult to treat in biomedical fields due to their protracted healing times. Bacterial infections, underlying medical disorders (such as diabetes and cancer), malnutrition, obesity, and smoking are a few of the causes of chronic wounds [1]. Hydrogels are 3D polymeric networks that can absorb water or biological fluids and can be chemically or physically cross-linked [2-4]. Hydrogels have recently gained a lot of interest for their use in tissue engineering, drug delivery, self-healing, and biosensors [5]. Methylcellulose (MC) is hydrophilic, which allows it to absorb wound exudate and keep the wound moistened [6]. PVP is a non-toxic, water-soluble, film-forming, and non-hazardous polymer [7]. The US Food and Drug Administration has recognized it as a safe and secure polymer for conducting biological experiments in the pharmaceutical and biomedical fields [8]. PVP wound dressings are developed by crosslinking PVP with PEG and agar and sterilizing them [9]. Along with hydroxyethylcellulose, CMC in its sodium salt form (Na-CMC) was exploited (HEC). Due to its superior mechanical properties, swelling ability, inexpensive cost, and greater number of constituents than pure CMC hydrogels, PVP/CMC blend hydrogel has a significant potential for application as a wound dressing [10,11]. PVP/CMC and PVP/CMC/BA hydrogels might work well for treating and healing wounds [12,13].
Maleic acid (MA) is a biodegradable and biocompatible crosslinker that can be used to create new functional biomaterials [14]. The methylcellulose/ Hyaluronic/ AgNPs hydrogels showed good antibacterial activity as wound healing material [15]. Antibacterial hydrogel films are required to regulate and sustain drug release in wounds. Cellulose derivatives loaded with metal and metal oxide nanoparticles are antibacterial and mechanically strong. The prepared nanocomposites were effective against both S. aureus and E. coli bacteria [16]. Zinc oxide (ZnO) nanoparticles are exploited in cosmetics and food packaging because of their natural antibacterial properties. The key advantages of adopting ZnO nanoparticles (ZnO NPs) over Ag nanoparticles were their low cost and colorlessness [17,18]. In wounds, zinc oxide nanoparticles inhibit S. aureus and E. coli germs. By boosting cellular immunological activity, hydrogels create a moist and hydrated environment for wound healing [19]. Infection of the wound can result in effusion, delayed healing, and abnormal collagen deposition. E. coli and S. aureus bacteria are common pathogens. These bacteria form enormous colonies within the body [20]. Zinc oxide nanoparticles improve wound healing. Wound healing using zinc oxide nanoparticles ZnO NPs is both cheap and safe [16].
Existing wound dressings have issues such as a lack of porosity, less flexibility, a tendency to stick to the wound surface, and a slower healing process (skin migration, connective tissue synthesis, and blood vessel formation); a poor ability to kill bacteria, difficulty in removing the wound dressing after healing, and the ability to cause allergic reactions. By combining biopolymers, synthetic polymers, and antibacterial nanoparticles to create hybrid-based wound dressings with improved properties, such as providing a moist environment to the wound bed, absorbing wound exudates due to its porous network, promoting good gaseous exchange useful in inhibiting bacterial growth, inducing epithelization and cell migration, and supporting tissue regeneration, they are useful for wound healing applications. To our knowledge, the formation and characterization of MC/PVP/ZnO nanocomposites have not yet been studied. Therefore, the aim of this research is to develop the biogenic ZnO NPs incorporated MC/PVP Bio-nanocomposites for wound healing applications.
Materials
Methylcellulose (MC) was purchased from HiMedia, India. PVP, maleic acid, and zinc acetate were purchased from SRL, India, and DPPH from Sigma Aldrich, USA.
Biosynthesis of ZnO Nanoparticles
50 grams of neem leaves were collected from the Rajshahi University campus and rinsed with distilled water. The neem leaves were boiled in 200 ml of distilled water for 20 minutes at 65 °C. After being agitated in a magnetic stirrer until the colour changed from orange to brown, the aqueous extract was filtered. To prepare ZnO nanoparticles, 50 mL of extract was mixed with 50 mL of zinc acetate. The solution was thoroughly agitated with a stirrer, and a white precipitate was obtained by adding 10 ml of 0.5 M NaOH while maintaining pH 7.0. After centrifugation, the ppt was dried at 65 °C in an oven and was calcined in a muffle furnace for 2 hours at 450°C [21,22]. ZnO nanoparticles formation reaction is as follows-
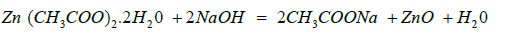
Preparation of MC/PVP/ZnO Nanocomposites
The hydrogel films were prepared using the solution casting method. Firstly, two solutions of 5% (w/v) MC and 5% (w/v) PVP were prepared separately. The solutions were then blended maintaining an 80:20 v/v ratio, and 10% (based on the total polymer matrix) maleic acid was added. The mixture was then heated for 70 minutes at 85 °C with gentle stirring. Using the same method, composites were made by gradually adding (1 mg/ml in water) suspensions of varying concentrations of ZnO NPs (2, 4, and 6 %)) to the MC/PVP solution while stirring. Then, at 25°C, it was sonicated for 10 minutes. The homogenous MC/PVP/ZnO suspensions were put into a transparent petri dish and allowed to evaporate for 72 hours at room temperature. Then transferred it to the Freeze drier for drying [14]. The reaction mechanism of this preparation is presented in Figure 1.
Gel Content
The gel fraction of hydrogels was determined by immersing the sample in distilled water at room temperature and measuring the sample's insoluble portion.
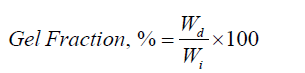
Where, Wd is the weight of the dried hydrogel after extraction, and Wi is the initial weight of the dried hydrogel [23].
Swelling Behaviour
A dried sample (1cm × 1cm) was immersed in water at 25°C and withdrawn at predefined time intervals. Surface water is removed using filter paper. Swollen hydrogels were weighed, and the percentage of water uptake capacity was measured by the equation below:
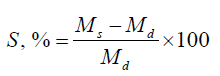
where S is the equilibrium water absorbency (%); Ms and Md are the weight of swollen hydrogel (g) at the time (t), and dry hydrogel (g), respectively [24]. Moreover, the capability to absorb water in simulated gastric fluid (SGF) at pH 1.2 and phosphate buffer solution (PBS) at pH 7.4 was assessed. The data represented the average results of three separate tests [25].
Water Vapour Transmission Rate (WVTR)
The European Pharmacopoeia (EP) standard was employed in the determination of the Water Vapour Transmission Rate (WVTR). A 35 mm inner diameter container with 25 ml of distilled water was filled with hydrogel film samples (3 mm thick), which were then cut into circular pieces and taped to the mouth. After that, the bottles were maintained in an oven with a 35°C temperature and a 35% relative humidity level. The WVTR (g/m2-day) calculation formula is provided below.
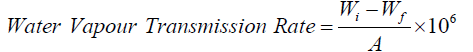
where, A is the permeation area of samples, and Wi and Wf are the initial and final weight of bottles, respectively. The experiment was conducted at 35℃ as the end application of the hydrogel on human skin, which has a temperature of 37.2℃ [26].
ATR-FTIR Analysis
In order to analyze MC/PVP hydrogel and MC/PVP/ZnO nanocomposites, ATR-FTIR spectroscopy had been used. A Perkin Elmer FTIR spectrophotometer (Model: Paragon 500, PerkinElmer, UK) with a sophisticated orbit Attenuated Total Reflectance (ATR) accessory was employed to record the spectra in the 4000-400 cm-1 region. The diamond crystal is mounted on the ATR. ATR-FTIR spectrum measurements were made in transmittance mode [27].
UV-Visible Spectroscopy
UV-Vis spectroscopy is an analytical procedure that measures the number of discrete wavelengths of UV or visible light that a sample absorbs or lets through to a reference or blank sample. Spectroscopy is since when chemical compounds take in ultraviolet or visible light; they make different spectra. For the UV-Vis test, a suspension of 1 mL of ZnO NPs was made and sonicated at 6000 rpm for 10 minutes. From 200 to 800 nm, the UV-Vis spectra were taken. UV-visible spectroscopy was also employed to evaluate the DPPH free radical scavenging activity [28].
Dynamic Light Scattering Analysis (DLS)
The particle size distribution of biosynthesized ZnO NPs was achieved by dispersing 10 mg of ZnO NPs in 100 ml of distilled water and sonication for 30 min then tested via (NICOMP 380 ZLS, Dynamic light scattering (DLS) apparatus (PSS, Santa Barbara, CA, USA) [29].
Hydrogel Porosity
Hydrogel porosity (%) was calculated using the following equation:
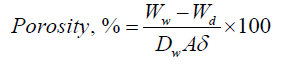
Where Ww is the weight of the wet sample, Wd is the dry sample weight (g), Dw is the density of pure water (g/cm3), A is the area of membrane in the wet state (cm2), and δ is the thickness of membrane in the wet state (cm) [30].
Field Emission Scanning Electron Microscopy (FESEM)
The surface morphology of the prepared samples was studied by FESEM images. To investigate the dispersion of nanoparticles within the polymeric matrix, field emission scanning electron microscopy (FESEM) was used with an EDX detector at an accelerating voltage of 5 kV.
Energy Dispersive X-ray Measurement (EDX)
The elemental composition of materials can be determined using the X-ray technique referred to as dispersive X-ray analysis (EDX), which is attached to SEM or TEM instruments. The information produced by EDX analysis consists of spectra with peaks corresponding to the constituent elements of the material understudy and is additionally feasible for image analysis and elemental mapping of the sample [31].
Assay of Antibacterial Activity
The resulting films were then cut into a 3 mm diameter disk. The films were placed on the culture surface of Escherichia coli and Staphylococcus aureus bacteria on the agar medium. After 24 hours of incubation at 37°C, the zones of inhibition around the disc were measured in millimeters [32].
Antioxidant Activity Test
The 2,2-diphenyl-1-picrylhydrazyl radical was used to test the antioxidant activity of bio-nanocomposite films. At 517 nm, the samples' absorbances were measured.
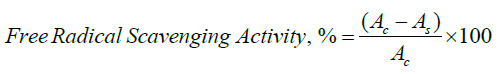
where Ac and As were the absorbances of DPPH of the control and test film, respectively [33].
Cytotoxicity by Hemolytic Potentiality Test
The prepared bio-nanocomposites were tested for hemolytic activity using fresh sheep blood with saline adding trisodium citrate as a blood anticoagulant, incubated at 37℃, taken distilled water for 100% hemolysis, and saline solution for 0% hemolysis. The absorbance of the supernatant at 545 nm was measured, and the hemolysis was estimated as follows:
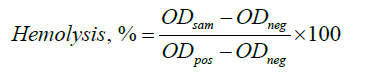
Where ODsam, ODneg and ODpos are the adsorptions of the sample, negative control, and positive control, respectively [34].
Biodegradation Study
The dried samples with 4×4 cm2 dimensions were weighed to determine their initial weight (Wi) before being immersed in deionized water for 24 hours. Natural degradation of the samples was observed for 4 weeks, following which samples were taken from the soil, cleaned to remove mud from the surface, and dried at 80°C for 8 hours.
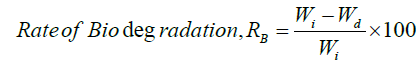
Where Wd is the dried weight of the hydrogel. Wi is the initial weight of the hydrogel; RB is the rate of biodegradation of the sample [35].
Gel Content
Gel content is an important characteristic since it controls the amount of cross-linking that occurs in the hydrogel network. A sol-gel conversion of a finite crosslinked polymer into an infinite number of molecules results in a hydrogel. The portion of the hydrogel that is left over after the sol has been removed is referred to as the gel content, and a higher gel content % indicates a stiffer hydrogel. The gel content of the prepared MC/PVP hydrogel was 76% and it decreased to 70% gradually with the addition of 2, 4, and 6% ZnO NPs to the polymer matrix.
Swelling Behavior
The ability to absorb fluids (blood plasma or serum) from wounds is one of the primary properties of hydrogel wound dressings. The swelling behaviour of the hydrogels was evaluated using distilled water rather than blood (because blood plasma contains around 90-92% water), and at room temperature, the dried hydrogel sample was allowed to expand in distilled water. As depicted in Figure 2, there was a fast swelling for the first 100 minutes, followed by a slower continuous swelling for 270-360 minutes (for equilibrium swelling). For MC/PVP without ZnO NPs, the maximum swelling degree reached was 1610%, and it tended to decrease as the proportion of ZnO NPs in the polymer matrix increased, as depicted in Figures 2a-2d. Since the ZnO NPs occupy the pores of the gel, there is less space for water to attach to the polymeric framework.
As shown in Figure 3 the water uptake behaviour of the films was tested in two solutions with pH values of 7.4 (Phosphate Buffer Saline, PBS) and 1.2 (Stimulated Gastric Fluid, SGF) to test the pH sensitivity of hydrogels. All samples absorbed more water when the pH was higher, such as 7.4. The electrostatic repulsive forces between negatively charged sites (COO-) result in an increase in the water absorption capacity when the pH is higher than the pKa of carboxylic groups (pKa 4-5) [36]. Low pH values cause most carboxylate anions to protonate. Lower levels of water absorption occur when the electrostatic repulsion between carboxylate groups is reduced. Additionally, the fact that there are more hydrogen bonding connections between carboxylate groups, which implies a larger physical crosslinking, may be the cause of the hydrogel films' lower capacity to absorb water [37].
Water Vapour Transmission Rate Test
High WVTR results in the development of scars, while low WVTR leads to the formation of exudates and enhanced bacterial growth. The WVTR values for MC/PVP, MC/PVP with 2, 4, and 6% ZnO NPs incorporated hydrogel films were 2450, 2345, 2286, and 2215 g/m2-day, respectively. To prevent the wound from drying out and losing water, as well as the development of exudates in the wound bed, the ideal wound dressing should have a WVTR of 2,000-2,500g/m2-day. Thus, our synthesized nanocomposites are best suited for use as wound dressings.
ATR-FTIR Analysis
FTIR spectra of pure MC, PVP, MA, biogenic ZnO NPs, and MC/PVP hydrogels with and without ZnO NPs addition were shown in Figure 4. Peaks in the 3300 cm-1 - 3500 cm-1 wave number range for all samples reveal the existence of hydroxyl groups (-OH), which imply hydrophilic properties and the ability of the hydrogel to absorb water, and the peaks in the 2800-3000 cm-1 wave number range for all samples indicate C-H stretching vibrations. The absorption peaks that appeared at 1430 cm-1 showed the presence of lignin C=O stretching and more intense vibrations at 1060 cm-1 indicate the presence of ether (C-O-C), 1430 cm-1 for -CH2- wagging in MC. PVP spectra could be stated as 1,640 cm-1 for C = O vibration. 1281 cm-1 for C-N stretching vibration, and maleic acid 1704 cm-1 for C=O stretching. Hydrogel without ZnO NPs showed a new peak at 1741 cm-1 indicating an esteric group & hydrogel with ZnO NPs shows also esteric bond at 1745 cm-1 and a new peak for the presence of ZnO NPs particles at 432 cm-1. As a consequence, it could be concluded that the chemical process of crosslinking between MC and maleic acid had been executed successfully.
UV-Visible Spectroscopic Analysis
As illustrated in Figure 5, UV-Visible spectroscopy was used to investigate the optical characteristics of neem leaf extract-assisted ZnO NPs. The absorbance peak at 372 nm demonstrates that biosynthesis was used to produce ZnO NPs. This is due to the intrinsic band gap absorption of ZnO, caused by electron transitions from the valence band to the conduction band (O2p-Zn3d). The evaluated value is less than that of bulk ZnO, which is 380 nm, and there is a blue shift in excitonic absorption, indicating that bulk metal oxides have a large band gap and less ability to interact. When their sizes are decreased, however, they become more responsive, and their ability to interact can be deduced from how well they reflect and absorb light.
Dynamic Light Scattering Analysis (DLS)
DLS is a new method that is widely used to estimate the hydrodynamic diameter of nanoparticle suspensions based on the Brownian motions of the particles. For the measurement, ZnO NPs in water were used. Figure 6 shows that the ZnO NPs that were made are stable. From the histogram study, particles with a 50.7 nm diameter made the most of the particles' volume.
Hydrogel Porosity
The pore structure of the nanocomposite hydrogel was evaluated. When ZnO NPs content is 0 wt%, sparse pores can be observed and the porosity is 88%, With increasing of ZnO NPs to 2 to 6%, the porosity decreases to 80%. The dressings' high porosity helps to absorb more exudate from a wound surface and prevent wound infection caused by exudates.
Field Emission Scanning Electron Microscopy
In Figures 7a-7c, as FESEM images, MC show the crystalline and fibre type morphology, while hydrogel without ZnO NPs showed a homogeneous blended structure and confirms crosslinking among MC, PVP and maleic acid successfully. FESEM images of the cross-section of the MC/PVP/ZnO composite film revealed that there were no voids in the nanocomposite indicating that the fillers are compatible with the polymer matrix and intermolecular binding. It assured the presence of ZnO NPs in the hydrogel with hydrophobic interaction. Energy-dispersive X-ray Analysis (EDX). The EDX analyses and the elemental composition of prepared hydrogels with ZnO NPs in both mass and atom% are listed in the following Table 1. The presence of N and Zn atoms indicates the existence of PVP & ZnO NPs in the prepared bio-nanocomposites.
Antibacterial Activity of the Prepared Composites
The antimicrobial activities of the MC/PVP, MC/PVP/ZnO (2, 4 and 6%), and antibiotic (control) are determined by testing them against Escherichia coli and Staphylococcus aureus bacteria. The results from Table 2 showed that prepared hydrogels are more effective against E. coli than S. aureus bacteria and the zone of inhibition increases with the increase of ZnO NPs% in the hydrogel structures. That is to say, ZnO NPs have strong antibacterial activity.
Antioxidant Test of the Prepared Composites
The results from Table 3 showed that the DPPH free radical scavenging activity of composites rises with the increasing conc. of ZnO NPs (0.2 mg/ml—35 %; 0.4 mg/ml-40.5 %; 0.6 mg/ml-46.2 %) in the polymer matrix and these results are close to the DPPH RSA% of ascorbic acid.
Effect of ZnO NPs on Blood Compatability
The hemolytic activity of MC/PVP and 2, 4, and 6% ZnO NPs incorporated bio-composites were calculated as 1.2, 1.75, 2.25 and 2.82% which showed negligible hemolytic properties according to the American Society for Testing and Materials. So, the prepared bio-nanocomposites were considered nonhemolytic materials.
Biodegradability Test of the Prepared Composites
In Figure 8, the MC/PVP film was disintegrated 100% in 28 days but for 2, 4, and 6% ZnO NPs incorporated nanocomposites after 28 days, the weight loss% was 84, 72, and 58%. So, the prepared hydrogels are moderately biodegradable.
The ZnO-loaded MC/PVP nanocomposite hydrogels were successfully prepared by the solution casting method. FTIR, FESEM and EDX results supported the presence of ZnO nanoparticles in prepared bio-nanocomposites. The swelling ratio was maximum for MC/PVP gels, but it decreased with increasing of ZnO NPs. It demonstrated antibacterial activity against E. coli and S. aureus bacteria and more ZnO NPs loaded composites scavenged more DPPH free radicals. Based on the results, all samples are biodegradable, non-toxic, water vapour permeable, and porous. So, the novel MC/PVP/ZnO bio-nanocomposites would be a better alternative to the traditional wound dressing.


