Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Firoz Ahmed1,2*, Md. Sofiuzzaman1, Abu Raihan1, Md. Monirul Islam1, H. Jahan Kadri1,2, Md. Ahsan Habib1 and Md. Ibrahim H. Mondal1*
Received: February 16, 2023; Published: March 21, 2023
*Corresponding author: Firoz Ahmed, Polymer and Textile Research Lab, Department of Applied Chemistry and Chemical Engineering, Rajshahi University, Rajshahi-6205, Bangladesh, BCSIR Laboratories Rajshahi, Bangladesh Council of Scientific and Industrial Research, Rajshahi, Bangladesh
Md. Ibrahim H. Mondal, Polymer and Textile Research Lab, Department of Applied Chemistry and Chemical Engineering, Rajshahi University, Rajshahi-6205, Bangladesh
DOI: 10.26717/BJSTR.2023.49.007793
In this study, the hydrogel was synthesized by the free-radical graft copolymerization of polyvinyl pyrrolidone (PVP) and acrylamide (AM) monomers onto carboxymethyl cellulose (CMC) in the presence of N, N-methylene bisacrylamide as a crosslinker, and potassium persulfate as an initiator. Also, the hydrogel’s physical properties (e.g., degree of swelling, water absorption, desorption, and gel content) were determined. The FTIR study showed that the copolymer’s spectra had clear PVP and AM repeating unit absorption bands, which supported the idea that the PVP and AM monomers had been grafted onto the CMC backbone. In addition, the hydrogel’s surface shape was revealed using AFM spectroscopy. TGA results confirmed that the prepared hydrogel was thermally stable up to 232°C. Water absorption by the prepared hydrogel was 110%. The biodegradability of the synthesized hydrogel was also investigated, and it reached a maximum of 82% at 26 days. The results showed that the prepared hydrogel film would be a good material for biomedical applications and wastewater treatment.
Keywords: Grafting; Hydrogel; Carboxymethyl; Cellulose; Polyvinyl Pyrrolidone; Biomedical
Abbreviations: CMC: Carboxymethyl Cellulose; PVP: Polyvinyl Pyrrolidone; AM: Acrylamide; KPS: Potassium Persulfate; DS: Degree of Swelling; MBA: Methylene Bisacrylamide, WA: Water Absorption
Hydrogels are chemically or physically cross-linked 3D polymeric networks that can absorb a large amount of water or biological fluids due to the presence of hydrophilic groups [1]. Hydrogels are considered one of the most promising materials due to their swelling and de-swelling properties and wide range of applications [2]. Increasing interest in natural-based hydrogel has developed mainly due to the high hydrophilicity, biocompatibility, non-toxicity, and biodegradability of the biopolymers [3]. Hydrogel can be in a variety of forms as natural or synthetic, modified hydrogels, hydrogel composites, and nanocomposites hydrogel [4]. Based on crosslinking, the hydrogel can be classified as physical, or reversible and chemical, or permanent. In the physical gel, hydrophobic and charge interaction, electrostatic, ionic, and hydrogen bonds form between polymer chains. And the gel formation can be reversed. On the other hand, the chemical cross-linking method uses covalent bonding between polymer chains to produce permanent hydrogel [5]. Based on properties, hydrogel materials have received sky-interests in biomedical and technical applications, including wastewater treatment, agriculture, the food industry, soft robotics, drug delivery systems, soft contact lenses, and tissue engineering scaffolds [6-7].
Carboxymethyl cellulose (CMC) is a cellulose derivative with excellent film-forming ability. CMC locks the free water molecules inside its interstitial structure at the time of hydrogel formation. When added to water, CMC tends to disperse and create a clear colloidal solution, sometimes known as CMC gel. CMC gels have been the subject of intensive study because of their favourable properties, including low toxicity and immunogenicity, intelligent response, high biodegradability, and biocompatibility [8]. A large number of carboxymethyl groups on the CMC backbone has been widely used as a natural ingredient for hydrogels mostly because of these advantages [9]. Polyvinyl pyrrolidone (PVP) is a biocompatible synthetic watersoluble material that can be used in various fields like adhesives in a glue stick, additives, the technical process, and disintegrate, for a thickening agent. Carbonyl groups of PVP provide these unique characteristics. For this reason, Polyvinyl pyrrolidone (PVP) is a good candidate for hydrogel synthesis [10]. Acrylamide (AM) is commonly known as a small molecular weight monomer. It is a white odorless crystalline solid and soluble in water Acryl amide (AM) is used to manufacture various polymer binding agents, flocculating agents, cement, sewage/wastewater treatment, pesticide formulations, cosmetics, food packaging, plastics products, paper productions, potting soil, and so on [11]. The aim of this study was to synthesize CMC-based hydrogel by using PVP and AM as a monomer, potassium persulfate as an initiator, and N, N-methylene bisacrylamide as a crosslinker by simple and easy free radical graft copolymerization.
Materials and Methods
Materials: Sodium carboxymethyl cellulose (Na-CMC, Pure), acrylamide, polyvinyl pyrrolidone, hydrochloric acid, and sodium hydroxide were purchased from BDH (England). All the reagents were analytical grade and used without further purification.
Preparation of the Hydrogel
At first, one gram of carboxymethyl cellulose (CMC) was dispersed in 30 mL of distilled water in a 250-mL four-necked flask equipped with a mechanical stirrer, a reflux condenser, and a thermometer. The reactor was immersed in a water bath at 60°C and kept for 1h under continuous stirring. After a certain time solution of potassium persulfate (KPS) was added to the solution of CMC and it was gently stirred for 15 minutes to facilitate free-radical formation on the CMC backbone. 1g of polyvinyl pyrrolidone (PVP) was dissolved in 15 mL of distilled water. Again, 1g of acrylamide (AM) was dissolved in 15 mL of distilled water. Then the solution of PVP and AM was added to the flask. The crosslinker N, Nʹ Methylene bisacrylamide (MBA, 10% of total monomer) was added just after adding the monomers. The reaction solution was heated by the water bath to the desired temperature to start the polymerization with constant stirring at 60°C for 2.0 h. After that, we obtained the hydrogel. The obtained product was cut into small pieces and dried in a vacuum oven at 70°C for 24.0 h.
Removal of Homopolymer from Hydrogel
The product sample was purified by extracting the homopolymer of polyacrylamide and polyvinyl-pyrrolidone from the crude product by washing with water and acetone mixture (70:30). The procedure was repeated three times. The graft copolymer was finally washed with pure acetone and allowed to dry in air for 24.0 h at room temperature.
Determination of Equilibrium Swelling of Hydrogel
The hydrogel sample was immersed in 250 mL of distilled water at room temperature for 24.0 h to reach swelling equilibrium. The swollen gels were separated from unabsorbed water by filtering through a 100-mesh screen and then drained for 10 minutes. After weighing the swollen gels, the swelling ratio of the hydrogel (Qeq, g/g) was calculated using the following equation:
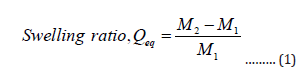
Where, M1 and M2 are the weights of the hydrogel sample and the swollen gel, respectively. The procedures were repeated 3 times repeatedly and the mean value was taken [12].
Swelling Behavior of Hydrogel
Gel samples were dried to a constant weight and immersed in distilled water at room temperature for 24.0 h. The degree of swelling (DS) of the hydrogel can be calculated as
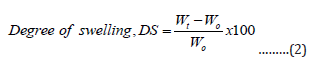
Where Wt is the weight of swelling hydrogel and Wo is the initial weight of dry gel [13].
Determination of Water Absorption of Hydrogel with Time
Hydrogel samples after extraction were dried to a constant weight and immersed in distilled water at room temperature. Hydrogel swells in water. At first, the weight of swelled gel was taken for 0, 4, 8, 12, and 16 h and it was continued after 24, 48, 72, and 96 h. After completion of the absorbing period, the hydrogels were taken out and the adhering water was removed using tissue paper. It was then weighed. The water absorption (Wa) is calculated as g H2O/g dry copolymer using the following equation:
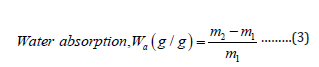
Electroencapsulation
Where, m2 means the weight of the sample with the absorbed water and m1 is the initial weight of the dried sample [14-15].
Determination of Water Desorption of Hydrogel with Time
The prepared hydrogel samples were weighted initially and then placed in an open environment for water desorption. At every 4 h interval, the weight of the samples was taken for the 0, 4, 8, 12, and 16 h, and, it was continued after 24, 48, 72, and 96 h until cons tant weights were established. Finally, the percentage of water desorption was calculated gravimetrically at room temperature.
Water desorption of the hydrogel can be calculated as,
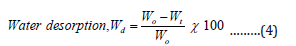
Where Wt is the weight of the dry hydrogel in air and Wo is the weight of the prepared hydrogel [16].
Gel Content of Hydrogel
The hydrogel samples were dried at 60oC in a vacuum oven to a constant weight. The gel content in the dried samples was estimated by measuring its insoluble part in the dried sample after immersion in deionized water for 48.0 h at room temperature. The gel fraction was calculated as follows
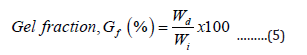
Where Wi is the initial weight of the dried sample and Wd is the weight of the dried insoluble part of the sample after extraction with water [17].
FTIR Test
FTIR spectra of hydrogels were recorded with Spectrum-100, FTIR Spectrum, and Perkin-Elmer. FTIR of the hydrogel sample was recorded using a KBr pellet within the frequency range 400-4000 cm-1.
TGA Test
Thermal analyses of all samples were carried out with Perkin- Elmer Simultaneous Thermal Analyzer (STA 8000, Netherlands). The tests were carried out between 30o to 700°C. The heating rate and the airflow rate were 20℃/min and 200 ml/min, respectively.
AFM Test
Atomic force microscopy is a powerful characterization tool for polymer science, capable of revealing surface structures with superior spatial resolution. Surface morphology was assessed using Atomic Force Microscopy (Park Systems, XE-70, South Korea).
Biodegradation
The biodegradability of the samples was determined by the soil burial method. Conventional garden soil (30°C, pH 6.0-8.5) was filled in plastic containers and moisture content was maintained at around 55%. The specimen (2 cm x 2 cm) was buried under the soil 10 cm from the topsoil. The water (20 mL) was poured at a regular time interval of 2 days. Every 7 days, the buried samples were carefully taken out, washed with distilled water, and dried at 50°C until the weight was constant before being weighed. The biodegradability was determined by measuring the loss of the specimens. The following equation to calculate biodegradation is as follows:
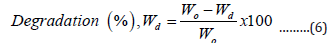
Where, Wd and Wo are the weights of dry products before and after degradation, respectively. WD is the rate of degradation of the hydrogel [18].
Optimization
The effect of the amount of CMC, polyvinyl pyrrolidone (PVP), and acrylamide (AM) on swelling of the hydrogel was studied by varying the amount of polyvinyl pyrrolidone (PVP) and acrylamide (AM) as shown in (Figures 1, 2 & 3). The effect of the amount of polyvinyl pyrrolidone (PVP) and acrylamide (AM) on swelling of the hydrogel was studied by varying the amount of polyvinyl pyrrolidone (PVP) and acrylamide (AM) as shown in (Figures 1 & 2). The maximum swelling was obtained at a 1:1 weight ratio of monomer. The increase of the swelling with increasing the amount of polyvinyl pyrrolidone (PVP) and acrylamide (AM) which can be attributed to the hydrophilicity of carboxylate groups. The subsequent decrease in swelling of the hydrogels can be ascribed to the low reactivity of the PVP monomer. This indicates that the monomer grafting onto CMC chains. In other words, the hydrophilic monomers in the polymer chains are reduced, which leads to a decrease in swelling.
The effect of CMC content on the swelling ratio was investigated and is shown in (Figure 3). With increasing content of CMC, the swelling ratio of the hydrogel first increased and then decreased. The swelling ratio enhanced with increasing CMC content until maximum absorption was achieved. As the CMC dosage was increased, the macromolecular radicals that can be used to graft with monomers were increased and the grafting efficiency was enhanced. As a result, the swelling ratio increased with increasing CMC content. Beyond this content, the viscosity of the reaction system was increased and the chain transfer reaction was restricted, which decreases the molecular weight of the graft polymer chains. Thus, the swelling ratio was decreased. So, it can be summarized that the maximum swelling was achieved with a weight ratio of 1:1:1 for polyvinyl pyrrolidone (PVP), acrylamide (AM), and carboxymethyl cellulose (CMC). The hydrophilicity of carboxylate groups was found to be responsible for the increase in swelling that occurred with an increase in the amount of polyvinyl pyrrolidone (PVP) and acrylamide (AM). This indicates that the monomer grafting onto CMC chains uniformly. More than 1:1:1 hydrophilic monomers in the polymer chains lead to a decrease in swelling due to more linking with CMC, which results in less capacity to absorb water in its interstitial structure. This is because the swelling is caused by more linking between the hydrophilic monomers in the polymer chains.
(Figure 4) showed that the water absorption of hydrogel was increased with the increase in soaking time. But after 24.0 h, the hydration of hydrogel was saturated. It absorbed water due to hydrogen bonding with water molecules. (Figure 5) showed that the water desorption of hydrogel increased with the increase in air drying time. But after 72 h, it becomes stable and no more desorption occurred. In that case, the gel has no more unbound water. The gel fraction of the hydrogel was 85% indicating that it was a good hydrogel. If the weight ratio of the monomers is more than 1:1:1, the gel fraction is about 100%, which means that the hydrogel is not homogeneous. So, there is a maximum cross-linker concentration, above which the gel is not uniform, and a minimum concentration, below which higher monomer doses are needed to make the expected gel. The change in cross-linking density in the hydrogels makes the swelling depend on both the doses of monomer and the cross-linker concentrations [19].
FTIR Analysis
The FTIR spectra of CMC, PVP, and hydrogel were presented in (Figure 6). In (Figure 6(a)), the peak at 3421 cm-1 showed –OH stretching of carboxylic acids. The peak at 1635 cm-1 indicated C=O stretching vibrations due to the presence of the carboxyamide group. The peak at 1400 cm-1 could be assigned to the CH2 bending modes of alkanes. In (Figure 6(b)), the vibrational band at 1662 cm1 corresponds to C=O stretching, and the bands at 1429 cm-1 are attributed to C-N stretching, respectively. In (Figure 6(c)), the existence of a rather sharp intense peak at 1733 cm-1 (C=O, carboxamide group) and at 1400 cm-1 is attributed to C-N, aromatic amine in FTIR spectra of the hydrogel were certain evidence of grafting of the copolymer.
TGA Analysis
In (Figure 7), the first step can be seen up to 1250C for both samples which were related to the loss of water from the samples. In the second step, the weight loss was due to the thermal decomposition of the polymeric chain. From (Figure 7), it is seen that Na- CMC decomposition started from 2640C but hydrogel started to decompose from 2320C. And from the TGA data, we can summarize that the produced hydrogel was thermally stable.
AFM Analysis
Atomic Force Microscopy is used for determining the surface properties of the hydrogel. (Figure 8), the AFM image of the amplitude of carboxymethylcellulose-g-(polyvinyl pyrrolidone-coacrylamide) hydrogels tend to show edges of the surface features of the prepared hydrogel. In (Figure 9), the AFM image of the phase of carboxymethylcellulose-g-(polyvinyl pyrrolidone-co-acrylamide) hydrogels reveals the presence of sub-micron building blocks with numerous visible pores on the surface. The presence of those visible pores on the surface reveals proof of superabsorbent hydrogel.
Biodegradation Study
One of the most important criteria for hydrogel’s use in biomedical applications is its ability to degrade over time. Introducing lipophilic connections like esters, anhydrides, and imines can actualize controlled biodegradation. Crystallinity and molecular weight loss are two morphological indicators of biodegradation. As time goes on, the physical qualities deteriorate, giving way to a product with less mechanical strength and simpler, harmless byproducts that are flushed out of the body with ease [20]. The biodegradability of hydrogel increased with increasing time. The biodegradation was calculated by using Equation 6. The degradation increased with time. From (Figure 10), it was seen that the maximum biodegradability of hydrogel was 82 % at a time of 26 days. So, it can be said that the prepared hydrogel is mostly biodegradable in nature.
The superabsorbent hydrogel was synthesized successfully by the free radical graft copolymerization technique. Grafting of (PVPco- AM) copolymer on the backbone of CMC was confirmed by FTIR and AFM. TGA results confirmed that the prepared hydrogel was thermally stable. Water absorption by the prepared hydrogel was 110% and the gel fraction was 85%. The biodegradability of the synthesized hydrogel was also calculated and it was the highest at 82% at the time of 26 days. All results implied that the prepared hydrogel film would be a promising material to use in biomedical and wastewater treatment.


