Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Bianca Araújo Fernandes Veras¹, Pamela Isabel Japura Huanca1, Igor de Sousa Oliveira1 and Sávio Benvindo Ferreira2*
Received: March 01, 2023; Published: March 21, 2023
*Corresponding author: Sávio Benvindo Ferreira, PhD in Pharmacology, Professor of Microbiology, Academic Unit of Life (UACV), Teacher Training Center (CFP), Federal University of Campina Grande (UFCG), 58900-000, Cajazeiras, Paraíba, Brazil
DOI: 10.26717/BJSTR.2023.49.007790
The term “microbiota” dates back to the beginning of the 20th century and refers to a large number of microorganisms, including bacteria, yeasts and viruses, which coexist in various parts of the human body (intestine, skin, lung, oral cavity). Growing evidence has confirmed that the microbiota is associated with the development of numerous diseases, including brain disorders and neurodegenerative diseases. A neurodegenerative disease that has been gaining prominence in the study of microbiota is Amyotrophic Lateral Sclerosis (ALS), which mainly affects the motor system. In this perspective, the present work consists of a narrative, qualitative, exploratory descriptive review with the objective of compiling knowledge about the relevance of the microbiota in the treatment of amyotrophic lateral sclerosis. This is a bibliographic search carried out in February 2023 in the PubMed and Virtual Health Library databases with the descriptors “microbiota”, “amyotrophic lateral sclerosis” and “treatment”, combined using the Boolean operator AND in the range of time over the past 5 years.
This research evidenced the relationship between microbiota and amyotrophic lateral sclerosis in four axes: relation between microbiota and central nervous system, the role of antibiotic use in ALS, microbiota composition in patients with ALS and fecal microbiota transplantation. The findings indicate that the microbiota may play a critical role in the development of amyotrophic lateral sclerosis, given that the influence of the microbiome in the protection of homeostatic microglia with the inhibition of the neurodegenerative phenotype, the presence of certain microorganisms influencing the progression of the disease and consequent degeneration, the role of antibiotic use in the progression of ALS, fecal microbiota transplantation as a therapeutic alternative for the treatment of this pathology and possible therapeutic strategies for ALS. However, research on the relationship between gut microbiota and ALS is still in its early stages, but the fact that the microbiota has been closely associated with other neurodegenerative diseases raises hopes for the future.
Keywords: Microbiota; Amyotrophic Lateral Sclerosis; Treatment; Central Nervous System; Fecal Transplant
Abbreviations: ALS: Amyotrophic Lateral Sclerosis; FALS: Family Amyotrophic Lateral Sclerosis; FMT: Fecal Microbiota Transplantation; CNS: Central Nervous System; ENS: Enteric Nervous System; VNS: Vagus Nerve Stimulation; CFT: Ceftriaxone; LPS: LipoPolySaccharides; NDD: Neurodegenerative Diseases; ROS: Reactive Oxygen Species; SCFA: Short-Chain Fatty Acids
The term “microbiota” dates back to the beginning of the 20th century and refers to a large number of microorganisms, including bacteria, yeasts and viruses, which coexist in various parts of the human body (intestine, skin, lung, oral cavity) [1]. Under healthy conditions, the microbiota exhibits stability, resilience and symbiotic interaction with the host. In this situation, the microbial community demonstrates high taxonomic diversity, high microbial gene richness, and stable core microbiota [2]. In addition, the composition of microorganisms varies from place to place, and the intestinal microbiota is considered the most important in maintaining the individual's health, especially due to its relationship with the microbiota-brain axis [3-5]. As humans are replete with such complex systems with trillions of microorganisms, advanced genetic sequencing technologies are focused on determining the relationship between changes in microbiota composition and various disease states. When subjected to external changes, the microbial balance can be affected, which leads to dysregulation of bodily functions [6].
From a pathological perspective, increasing evidence has confirmed that microbiota is associated with the development of numerous diseases, including brain disorders and neurodegenerative diseases [6]. The influence of the microbiota on the progression of neurological diseases is through the intestinal microbes that are involved in the regulation of brain function through their effect on the innate immunity of the host [7]. A neurodegenerative disease that has been gaining prominence in the study of microbiota is amyotrophic lateral sclerosis (ALS), which mainly affects the motor system, but extramotor manifestations are increasingly recognized. Such a pathology develops with loss of upper and lower motor neurons in the motor cortex, brainstem nuclei, and anterior horn of the spinal cord, and the symptomatology consists of progressive muscle weakness and atrophy. ALS has a focal onset, but spreads to different regions of the body, and causes respiratory muscle failure that normally limits survival to 2 to 5 years after disease onset [8].
Although many scientific advances have clarified the pathogenesis of ALS, there are still unresolved challenges regarding the efficient delivery of drugs to the central nervous system that hinder the treatment of this pathology. These challenges include the presence of the blood-brain barrier as well as the inherent characteristics of the drugs themselves. As a result, conventional drug delivery systems do not facilitate proper dosing of medications needed for functional recovery in patients with ALS [9]. Thus, highlighting the problem of developing an effective treatment and the high lethality of the ALS disease, this study proposes the contemplation of a state of the art on the relevance of the microbiota in the treatment of amyotrophic lateral sclerosis. In this context, its scope is to carry out a narrative review of the scientific literature, serving as a basis for future investigations.
Research Characterization
The work consists of a narrative, qualitative, exploratory-descriptive review. The review was used as a method to compile knowledge about new therapeutic strategies related to the microbiota that may help in the treatment of amyotrophic lateral sclerosis. In view of this, a bibliographical analysis of the scientific literature was carried out to collect the main relevant information on the subject, focusing on the published news. In the investigation, a guiding question was elaborated through the PICo strategy (Population/Interest/Context).
Conducting the Investigation
The study question was determined: “What is the relevance of the microbiota (P) as a therapeutic strategy (I), considering the context of amyotrophic lateral sclerosis (Co)?”. Then, the bibliographic search began in February 2023 in the PubMed and Virtual Health Library databases. The descriptors “microbiota”, “amyotrophic lateral sclerosis” and “treatment” were used as search terms, combined using the Boolean AND operator. The time interval of the last 5 years was determined. The selected works portray in their abstract or title that the text refers to the relevance of the microbiota in the neurodegenerative disease ALS.
Selection Criteria
The prioritized works were those published in the form of scientific articles in Portuguese, English and Spanish. After carrying out the inclusion process, articles that did not present the research results in full, duplicated, or that the body of the text did not match or answer the guiding question of the investigation were excluded from the sample. Subsequently, the selected works were read in full, highlighting the relevant information to contemplate the scope of this review.
Presentation of Findings and Synthesis of Information
After the individual study of the articles, the construction of the state of the art began, which aims to answer the research's guiding question. There was no need to resort to judges for the qualitative treatment of data, due to the type of methodology chosen. In addition, submission to the research ethics committee was not required, as the samples represent published and available data.
The search for an effective treatment for amyotrophic lateral sclerosis makes the study of the human microbiota attractive for the investigation of possible therapeutic strategies. In total, 21 articles were used for the elaboration of this research. It was observed that studies found significant changes in the microbiota of patients diagnosed with ALS and such changes can be used as a target for the development of future treatments [10-15].
Relation Between Microbiota and Central Nervous System
Gut microbiota communicates with the brain through a complex neurohumoral connection called the gut-brain axis, which includes: the Central Nervous System (CNS), the autonomic nervous system, the Enteric Nervous System (ENS), and the hypothalamus-brain axis. pituitary-adrenal and the immune system. The vagus nerve, as the main component of the parasympathetic nervous system, establishes one of the connections between the intestine, the brain and inflammation and represents an important link between nutrition and diseases [11,16]. In this context, an important ability of the intestinal microbiota was reported, which consists of the ability to shape microglia, the primary immune cell of the central nervous system. In patients with ALS, microglia transition from a homeostatic to a neurodegenerative (MGnD) phenotype and contribute to disease pathogenesis. Findings with mice suggest that the microbiota plays a protective role by restricting MGnD microglia. This sheds new light on the importance of disease-specific interactions between microbiota and microglia [10,17].
Indeed, the microbiota restricts neurodegenerative microglia to slow the progress of ALS by producing metabolites or peripheral immune system modulator cells that traffic to the CNS. Furthermore, altered immunity of the integrated Central Nervous System (CNS) can have destructive consequences, which include nerve cell death causing neurodegenerative disorders. Thus, the undesirable immune response affects progressive neurodegenerative diseases, such as Amyotrophic Lateral Sclerosis (ALS) [17-19]. In other words, it is defined that neurodegenerative disorders may be linked to the gut-brain-immune correlational system, because the gut microbiota may play a role in neurological diseases through modulation of immune responses in the central nervous system, altering endocrine signaling to the along the hypothalamic-pituitary axis and signaling directly through afferent nerves [10,18,19].
Furthermore, patients with ALS have been observed to have abnormal gastrointestinal systems, elevated intestinal inflammation, and dysbiosis. The ENS and smooth muscle automatism are unable to modulate the motor functions of the digestive tract, which provides an anatomical explanation for these clinical manifestations. This process is related to the fact that 70-80% of the total number of human immune cells are within the lymph nodes of the intestinal mucosa, which suggests the possible role of the microbiota in the ability to impact neuroimmune [19-21]. Although the etiology of ALS is still not well established, the gut microbiota may mediate the pathology of the disease, mainly due to pro-inflammatory gut microbiomes (Figure 1). Of note, the pro-inflammatory state of the gut leads to neural disturbances and, over time, to neurodegeneration, as the expression of pro-inflammatory cytokines contributes to progressive damage to the central nervous system and inhibits self-repair processes [13,14].
Figure 1 Source: TOLEDO (2022). Figure 1: A dysfunctional gut accompanies the progression of ALS, with increased bacteria, including E. Coil and enterobacteriaceae, leading to upregulation of damaging Reactive Oxygen Species (ROS) and eventually contributing to motor neuron death, which is a hallmark pathological manifestation of the disease.
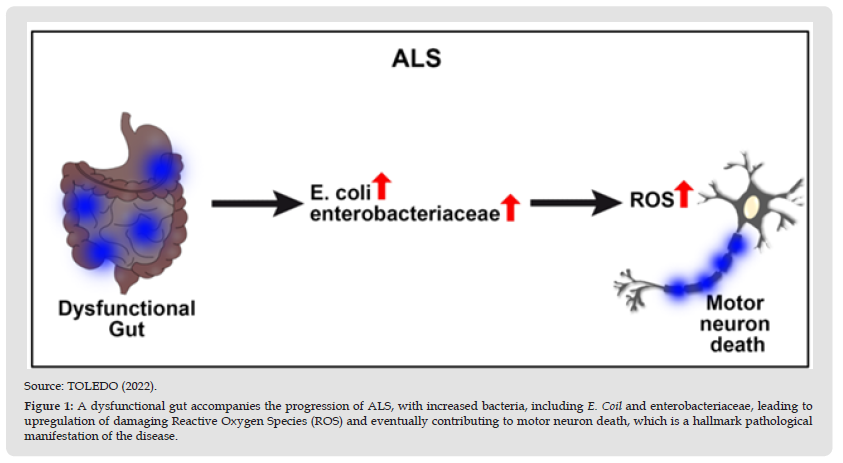
Likewise, a deleterious feedback loop occurs with the initial inflammatory insult, subsequently triggering pro-inflammatory immune components such as microglia, macrophages, neutrophils, and natural killers to invade brain tissue and create neurological dysfunctions [13,14]. In this sense, patients suffering from ALS have higher levels of pro-inflammatory cytokines and biomarkers in the cerebrospinal fluid and spinal cord, such as IL-8, IL-6, MCP-1, and the expression of CD1, CD40, among others. Pathologic bacterial expression of LPS and inflammatory cytokines, which are commonly associated with dysbiotic gut microbiomes, exacerbates chronic microglial activation [13]. Thus, the gut-brain axis may be the source of the pathophysiology of ALS in its inability to eliminate Reactive Oxygen Species (ROS) and other neurotoxic agents, which consequently increases motor neuron death. This specific pathological characteristic of amyotrophic lateral sclerosis, in addition to the pro-inflammatory state produced by intestinal dysbiosis, creates a health scenario where it is crucial to attend to both the neurological clinical manifestations and the imbalance that exists in the intestine [13,22,15].
Furthermore, since the direct line of communication between the gastrointestinal system and the brain is through the vagus nerve, a technique commonly used to treat a variety of disorders has emerged: Vagus Nerve Stimulation (VNS). Such a technique has the potential to modulate the enteric microbiota, allowing the investigation and possible treatment of neurological disorders in which the microbiota has been associated with the disease. Thus, one study investigated the effect of VNS in a mouse model of amyotrophic lateral sclerosis [11,16]. In this study, animals underwent ventral neck surgery to access the vagus nerve. However, it was observed that VNS did not alter gastrointestinal microbiota populations, indicating that this short intraoperative treatment is safe and has no lasting effects on GM. Future studies are needed to determine whether different stimulation parameters or chronic use of VNS affect GM profiles [16].
In addition, another finding of amyotrophic lateral sclerosis and the microbiota is about the familial typology of ALS, in which some advances in the treatment of the animal model C9ORF72 (one of the genes of amyotrophic lateral sclerosis) were obtained using a modulation of the microbiota, and this strategy has the great advantage of having an easy route of administration and a good safety profile. The experimental treatment landscape for FALS is rapidly evolving and the results are promising. This demonstrates the close relationship between the microbiota and the central nervous system [23].
The Role of Antibiotic Use in ALS
Amyotrophic lateral sclerosis is a genetically heterogeneous disease, in which the interaction between genetic inheritance and environmental factors is believed to play an important role. Familial ALS accounts for approximately 10% of cases, which results from genetic alterations in several genes, including superoxide dismutase 1 (SOD1). The remaining 90% of cases are sporadic, suggesting an important environmental component [10]. A study conducted in mice revealed that treatment with low-dose antibiotics worsened motor function and decreased survival in SOD1 mice, which is consistent with studies using high-dose antibiotics. When investigating changes in the microbiome, it was observed that antibiotics reduced Akkermansiae butyrate-producing bacteria, which can be beneficial in ALS. In this context, preclinical studies were also performed and reported an association between repeated use of antibiotics and an increased risk of ALS [10,17,24].
Furthermore, it was observed whether there were alterations in the immune cells residing in the CNS through the observation of spinal cord microglia. In this regard, it was found that antibiotics decreased homeostatic genes and increased genes for neurodegenerative diseases in SOD1 mice. Thus, antibiotic-induced changes in microglia preceded changes in motor function, suggesting that this may be contributing to disease progression [10]. In general, the microbiota can influence CNS neuronal health in different ways, directly producing neuroactive metabolites or toxins, or indirectly modulating the immune system. Another hypothesis is that the microbiota can affect the circulating levels of inflammatory cytokines that can damage the CNS, since studies show that the administration of some probiotics, such as Akkermansia muciniphila, can attenuate the symptoms of ALS exacerbated by antibiotics in mice, increasing the levels of nicotinamide in the CNS [10,17].
In contrast, it was observed that antibiotic-induced changes in mice suggest that the combination of genetic risk and microbiota dysbiosis is necessary to worsen antibiotic-induced motor function. In the study carried out, a neurodegenerative molecular signature was identified in the microglia of SOD1 mice, called MGnD, in contrast to a homeostatic microglial phenotype. As the gut microbiota may play a role in maintaining microglial function in homeostasis, antibiotics may decrease microglial homeostatic signatures. This leads to antibiotic-induced dysbiosis rather than total microbiota depletion [10,17]. The study showed that antibiotics initially depleted most endogenous microbiota populations and then led to an increase in antibiotic-resistant organisms. In this sense, antibiotic treatment did not affect the time of disease onset, but it did affect disease progression, potentially suggesting that administering antibiotics in the early symptomatic phase may also have a similar effect on the course of the disease [10,12]. In the mouse study, they identified several groups of antibiotic-depleted bacteria that may have beneficial roles in ALS. The Gram-negative anaerobe, Akkermansia was depleted at several time points. Akkermansia has recently been shown to improve disease in SOD1 mice pretreated with antibiotics, which is linked to nicotinamide production [10].
Antibiotics also depleted several members of Clostridial groups IV and XIVa, which are the main producers of butyrate in the gut. Two independent studies found that butyrate-producing bacteria were depleted in ALS. So, not only do antibiotics deplete protective bacteria, they can also select for pathogens that can make the disease worse. Recent human studies have suggested that antibiotics may contribute to the onset or progression of ALS disease. Thus, antibiotic treatment exacerbates the disease course in mice, and distinct commensal bacteria have been correlated with the severity of ALS [10,12,17]. However, not all antibiotics have harmful effects in amyotrophic lateral sclerosis. There are reports in the literature that beta-lactam and cephalosporin antibiotics, such as Ceftriaxone (CFT) and others, have demonstrated efficacy in the treatment and relief of symptoms of neurologically based diseases and experimentally induced neurological disorders, such as ALS. CFT has been reported to have neuroprotective and anti-inflammatory effects.
CFT pretreatment attenuates pro-inflammatory cytokines such as Nuclear Factor-κB, interferon-γ and/or tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in various neurological models [19]. Clinically, CFT, tetracycline and other beta-lactam drugs have been used to treat amyotrophic lateral sclerosis. The first identified use of CFT for the treatment of ALS was in 1988, when a study was conducted to explore a relationship between ALS and Lyme disease. Lyme, also known as Borrelia burgdorferi infection, has been correlated with ALS when anti-Borrelia antibodies have been found in ALS patients. Thus, a course of Ceftriaxone was attempted because the drug had recently been found to be active against Borrelia [19]. In that study, serum samples from patients with ALS were positive for anti-Borrelia Antibodies by anti-Borrelia immunofluorescent antibody titers. Halperin and associates confirmed that anti-Borrelia antibodies were not found infrequently in ALS cases. They examined and began treating with CFT a subset of nine patients at various stages of ALS disease progression who had anti-Borrelia antibodies. They found that three patients appeared to improve, three had disease progression, and three were apparently unaffected. This initial finding suggests that the temporal course of the disease has a strong influence on the pathogenesis and the results of the intervention [19].
Composition of the Microbiota of Patients With ALS
In patients with ALS, reduced relative microbial abundances were found in butyrate-producing Anaerostipes, Oscillibacter and Lachnospira, while that of glucose-metabolizing Dorea was significantly increased. Patients with ALS also have elevated plasma levels of lipopolysaccharides (LPS), which support neuroinflammation through activation of microglia. In addition to the differences in the intestinal microbiota, differences were also evaluated and found in the feces between patients with ALS and healthy ones [11,19,25,26]. In this theme, studies have found alterations in the intestinal microbiota of patients with ALS, analyzed a cohort of 37 patients with ALS versus 29 healthy controls and found a decrease in the abundance of microbial genes involved in the metabolism of nicotinamide and tryptophan. Another research sequenced the largest number of individuals with ALS to date (n = 68) versus healthy controls (n = 61) and found a decrease in butyrate-producing bacteria, Roseburia intestinalis and Eubacterium rectale. Furthermore, changes in butyrate-producing bacteria are consistent with a case study in which 5 patients with ALS had low levels of other butyrate-producing bacteria [10].
One of the findings of patients with amyotrophic lateral sclerosis is elevated levels of LPS in the plasma, this condition is called metabolic endotoxemia. It is suggested that this condition is related to chronic inflammation diseases, such as ALS. Therefore, this finding has attracted attention as a target for the prevention and treatment of chronic diseases [11,25]. As metabolic endotoxemia was first reported in mice fed a high-fat diet, research into its relationship to diets has been actively conducted in humans and animals. Changes in the composition of Firmicutes and Bacteroides due to excessive fat intake have contributed to metabolic endotoxemia in many reports and there are mucin-adherent bacteria thought to be involved in metabolic endotoxemia (e.g., Akkermansiae and Bacteroides) [25].
Furthermore, the role of gut microbiota as an environmental factor has been addressed in 2 metagenomic studies and in another study using polymerase chain reaction techniques. The last study quantified certain bacterial genera in 50 patients with ALS and in 50 age- and sex-matched controls, finding greater abundance of Escherichia coli and Enterobacteria and lower abundance of yeasts and Clostridium in the patient group [14,27]. In a previous study, published in 2016, a group of researchers used metagenomics techniques to compare microbial diversity in 6 patients with ALS and 5 controls, and found differences in the Firmicutes / Bacteroidetes (F/B) ratio and in some genera defined by the authors as beneficial and harmful microorganisms [27]. Furthermore, recent data suggest the new roles of gut dysfunction and the microbiota in the etiology and progression of ALS. One of these data found was that the intestinal microbiota can translocate to the bloodstream when intestinal vascular permeability is evident and metabolites triggered by bacteria can control glutamate and GABA, generating stress in the CNS. In this sense, the presence of LPS in blood circulation was detected in stressed rats [20-22].
Other research has shown that there are some differences in microbiota composition between patients with a distinct clinical phenotype. Indeed, patients with the classic phenotype had lower microbial diversity compared to others, and patients with milder disease progression had higher amounts of fecal Short-Chain Fatty Acids (SCFAs) [28]. Interestingly, supplementation with SCFAs such as butyrate restored microglia density and morphology in mice. Another benefit of butyrate supplementation is the restoration of GM diversity, greater homeostasis in gastrointestinal barriers and weight loss [14,19,26,28]. Notably, SCFAs, which are end-metabolic products of dietary fiber produced primarily by Bacteroidetes and Firmicutes. With regard to the rate of disease progression, patients with slow progression stand out from the rest due to their reduced diversity, lower F/B ratio and greater abundance of bacteria belonging to the Streptococcaceae family (in particular, the genus Streptococcus, which progressively decreases with increasing rate of progression) [28].
In addition, in recent studies, another characteristic related to the microbiota was observed, which was the fact that the high level of endotoxin/LPS in the plasma of patients with ALS is positively correlated with the activation of monocytes/macrophages in the blood. Indeed, low tolerable doses of LPS have been shown to be able to activate a neuroprotective effect on microglia, so there is a direct relationship between high levels of LPS and neurodegeneration via microglia [21]. In another perspective, a study observed that the comparison between individuals with ALS and a healthy group revealed a variation in the intestinal microbial composition with greater abundance of E. coli and Enterobacteria and low abundance of total yeast in patients. Polymerase chain reaction denaturing gradient gel electrophoresis analysis showed a cluster distinction between the bacterial profiles of patients with ALS and healthy subjects [13,29].
It is well known that the upregulation of specific pathological bacteria, such as E. coli and other Enterobacteria, accompanies the clinical manifestations of ALS and a worse prognosis for long-term survival. Thus, the effects of disease progression and bacteriotherapy on bacterial and yeast populations are ongoing [13,29]. Furthermore, the researchers decided to evaluate the microbiota of patients with ALS and their spouses. In that study, it was observed that the intestinal microbial communities of patients with ALS were more diverse and were deficient in Prevotella spp. compared to those of their spouses. Predictive analysis of microbial enzymes revealed that ALS patients showed decreased activity in several metabolic pathways, including carbon metabolism, butyrate metabolism, and systems involving histidine kinase and response regulators [14]. Thus, patients with amyotrophic lateral sclerosis reported in the study exhibit differences in their gut microbial communities compared to spousal controls and modification of the gut microbiome, such as through improvement of Prevotella spp. deficiency and/or alteration of butyrate metabolism may have translational value for the treatment of ALS [14].
Fecal Microbiota Transplantation (FMT)
Treating gut microbiota dysbiosis through microbiota restoration would have the potential to interfere with and delay the progression of ALS. Amyotrophic lateral sclerosis studies should be performed considering diet, microbiome, lifestyle, and gender difference. Studies that examine the microbiome along with gut pathogenesis will help determine when, where, and whether the microbiome and metabolites are critical to disease progression. Understanding the pathogenesis of ALS will provide innovative strategies for accurate diagnosis and better treatment for this challenging disease [20]. In this context, an alternative treatment for neurological disorders is Fecal Microbiota Transplantation (FMT), given that a different composition of the human intestinal microbiota compared to healthy controls has been reported in amyotrophic lateral sclerosis. Several studies suggest an important role of the gut microbiota in the pathophysiology of neurological disorders, suggesting that altering the gut microbiota can serve as a treatment strategy [30].
Thus, FMT treatment techniques for neurological disorders are planned or underway for amyotrophic lateral sclerosis [30]. From this perspective of treatment, a transplant of washed microbiota was performed, which stopped the deterioration of amyotrophic lateral sclerosis. The case report shows growing evidence suggesting a bidirectional link between gut microbiota and neurodegeneration. In it, a female patient with ALS underwent a flushed microbiota transplant (WMT), an enhanced fecal microbiota transplant (FMT), through a transendoscopic enteral tube during a 12-month follow-up [17,30]. Notably, a trend of microbial and metabolomic change consistent with disease state was observed. This case report shows for the first time direct clinical evidence of the use of WMT for the treatment of ALS, indicating that WMT may be the new treatment strategy to control this so-called incurable disease [17]. In this case, some bacteria successfully colonize or accumulate after WMTs.
The phyla Firmicutes and Verrucomicrobia, Prevotella spp. and Ruminococcus spp. were thought to be donor derived. Increased Firmicutes and decreased Bacteroidetes have a beneficial impact on CNS neuroendocrine and immunity, while a lower Firmicutes/Bacteroidetes ratio has been used as a marker of intestinal dysbiosis [17,27]. An important finding in this case comes from experience after the patient's condition was aggravated by antibiotic treatment for scalp trauma. Notably, intestinal microbial communities from patients with ALS were deficient in Prevotella spp. and Ruminococcus spp. compared to controls, which were increased after WMTs in this patient. In contrast, some bacteria with potential neurotoxic or pro-inflammatory activity, such as the Proteobacteria phylum, were obviously diminished. The clear finding is that WMT stopped the deterioration of ALS again [17]. Preliminary literature suggests that FMT may be a promising treatment option for several neurological disorders. However, the available evidence is still scarce and some contrasting results have been observed. A limited number of human studies have been performed or are ongoing, while for some disorders only animal experiments have been conducted. Large double-blind RCTs are needed to further elucidate the effect of FMT on neurological disorders [30].
Possible Treatment Options for ALS
Considering stem cells as a treatment for ALS may be an option, as if administered intravenously, they prefer to migrate to the intestine and not to the brain, reducing intestinal and brain inflammation. Another option is the transplantation of intestinal microflora from a protective environment, which suppresses systemic and neural inflammation [13]. As there is a strong relationship between intestinal dysbiosis and ALS, new therapeutic strategies are emerging aimed at modulating intestinal dysbiosis through prebiotics, probiotics, symbiotics or dietary interventions, butyrate supplementation and Fecal Microbial Transplantation (FMT) in the treatment of NDD (Neurodegenerative Diseases) [13,22].
In the case of probiotics, they provide resistance against damage caused by pathogenic bacteria to neurodegeneration and provide synthesis of metabolites that benefit neuroprotection, but also influence some behavioral disorders such as anxiety, depression and chronic fatigue syndrome. In this perspective, there is also treatment with bacteria that produce short-chain fatty acids, which improves the inflammatory microbiome [13,19]. It was previously believed that the diversity of bacteria in the gastrointestinal (GM) Microbiota predicts good health, but it has been proven to be the opposite, because gains or losses trigger disease and aging, that is, the diversity of bacteria in the GM does not ensure good health [26]. In addition, it is known that in the production of drugs, amyotrophic lateral sclerosis presents some barriers, but there is an experimental drug that delays the advance of the onset of motor symptoms, providing protection to motor neurons and increasing plasma levels of β- hydroxybutyrate, which is called Triheptanoin [19]. Thus, as in ALS it is possible to differentiate some specific characteristics, such as microbes and inflammatory cytokines, several studies aim to find the profile of inflammatory cytokines and the characteristic microbial composition of the disease so that they can be biomarkers and, finally, understand the heterogeneity of ALS [ 27].
It is clear that although the microbiota may play a critical role in the development of ALS, studies involving innate immunity and intestinal changes in the initial pathology of the disease are limited. Studies on microbiota alterations with pro-inflammatory serum cytokines and LPS in patients will help in the early diagnosis of the disease. However, research on the relationship between gut microbiota and ALS is still in its early stages, but the fact that gut microbiota has been closely associated with other neurodegenerative diseases raises hopes for the future. Thus, there is a great relevance of studying the microbiota in the development of the treatment of amyotrophic lateral sclerosis, as it has been shown in research that there is a change in the composition of the microbiome of patients with ALS and the use of antibiotics has a negative impact on the disease. Therefore, it is necessary that rigorous studies are carried out to determine the real effects of changes in the microbiota in the development of amyotrophic lateral sclerosis, and thus it is possible to outline effective therapeutic strategies for the disease considered incurable.


