Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Mariam Hammoud2, Abbas Sabbah1,2, Zahraa Allaw1 and Hassan Rammal1,2*
Received: February 17, 2023; Published: March 15, 2023
*Corresponding author: Hassan RAMMAL, Faculty of Agronomy, Platform for Research and Analysis in Environmental Science, Lebanese University, Hadat Campus, Beirut, Lebanon
DOI: 10.26717/BJSTR.2023.49.007779
Lebanon is distinguished by a great wealth of plant species especially with medicinal properties. In fact, 2607 wild species of which 92 are endemics can be found in only 10452 km2. Phenolic compounds are an essential part of the human diet and are of considerable interest due to their antioxidant properties and potential beneficial health effects. The objectives of this study is to extract and to purify polyphenols and alkaloids from the Lebanese Anacyclus nigellifolius Boiss and to evaluate their antioxidant using the DPPH test, their anti-tumor property on different cancer cell lines using the MTT test, and finally their antibacterial activities on gram positive and negative bacteria. The obtained results indicated that extracted polyphenols and alkaloids have an IC50 of 16 μg/mL and 22 μg/mL respectively Also, the antibacterial activity of polyphenols (MIC=1.125mg/mL) exceeded that of alkaloids (MIC=1.75 mg/mL) against E. coli and S. epidirmedis. Finally, these two compounds have exerted an antiproliferative effect on the viability of HCT-116 and HT-29 cells.
Keywords: Anacyclus Nigellifolius Boiss; Polyphenols; Alkaloids; Biological Properties
Abbreviations: MIC: Minimum Inhibitory Concentrations; MBC: Minimum Bactericidal Concentrations; CLSI: Clinical Laboratory and Standard Institute; OD: Optical Density; MBPC: Minimal Biofilm Prevention Concentration; SD: Standard Deviation
Due to the fact that herbal remedies contain naturally occurring active chemicals that can support human health, the general public is now more interested in them than in synthetic medications (Dmitri, et al. 2015). It has been estimated that as many as 75% to 90% of the world’s rural people rely on herbal traditional medicine as their primary health care (Akerele, et al. [1]). These herbal treatments are used for managing common ailments like fever, infections, sleeplessness, colds, cough, anxiety, arthritis, premenstrual syndrome, weakness, and cancer on a self-prescribed basis (Martínez-Graciá, et al. [2]). Herbs and spices have strong biological effects that save the body from harm brought on by oxidative stress created by free radicals and then postpone the onset of many oxidative stress-related disorders like cardiovascular disease, cancer, diabetes, and Alzheimer's (Shahidi, et al. [3]). They also, possess antimicrobial activity against several different germs, yeast, molds and viruses (Negi [4]). In actuality, active phytochemicals such carotenoids, flavonoids, polyphenols, terpenoids, sulfides, lignans, and plant sterols are what cause these biological actions. Phenolic compounds are classified as primary antioxidants which are mainly free radical scavengers that delay or inhibit the initiation step or interrupt the propagation step of lipid oxidation, thus decreasing the formation of volatile decomposition products (e.g., aldehydes and ketones) that cause rancidity (Alamed, et al. [5]). Currently, one of the complementary and alternative therapies that cancer patients utilize the most frequently is herbal therapy.
Several appropriate plants and plant extracts have been shown in study after study to have greater ability to kill cancer cells than common treatments (Edwards [6]). Some studies have shown that as many as 6 out of every 10 people with cancer (60%) use herbal remedies alongside conventional cancer treatments (Jemal, et al. [7]). The abundance of plant species in Lebanon, especially those with therapeutic qualities, sets it apart from other countries. In fact, just 10452 km2 can support 2607 wild species, of which 92 are endemic. In this study, we used the DPPH assay to first look at the in vitro antioxidant activity of polyphenols and alkaloids derived from this plant. Then, to determine the antiproliferative capacity of these extracted polyphenols and alkaloids on two cancer cell lines HCT-116 and HT-29 using the MTT assay and to evaluate their antibacterial activity on different bacterial strains. Finally, the antibiofilm activity of these polyphenols and alkaloids on E. coli biofilm was determined.
Plant Collection and Preparation of Powders
nigellifolius was gathered from the south region of Lebanon at 300 m of altitude. The biological authentication was carried out by the Professor George Tohme, the president of the Lebanese C.N.R.S. After being cut off from the plant, leaves and stems were thoroughly cleaned, then dried away from sunlight in the shade at room temperature. They were rotated throughout the drying process to promote homogenous drying. They were then maintained in a container away from light, heat, and moisture for later usage after being ground with a grinder to create a powder form.
Extraction of Polyphenols (Chart 1): The method of Bharadwaz and Bhattacharjee [8] was used for the extraction and purification of polyphenols. First, 100 g of nigellifolius powder was macerated in 750 mL of 70% methanol in a water bath for 30 minutes. The methanol was then entirely removed from the solution using a rotary evaporator at 50oC after the solution had been filtrated. After adding 500 mL of ethyl acetate, the solution received 0.3g of ascorbic acid to stop oxidation before being allowed to separate in a separatory funnel. During separation, the solution was split into two portions: the upper yellow portion, which included polyphenols and ethyl acetate, and the dark brown portion (containing water and other components). In order to collect and analyze the powder of remaining polyphenols in the flask, the upper yellow portion was collected, and the ethyl acetate was completely evaporated under lower pressure in the rotary evaporator (Chart 1).
Extraction and Purification of Alkaloids (chart 2): Dry powder (200 g) was first dissolved in 500 mL of ethanol (80 %). Then it undergoes reflux for 2 hours. The ethanol was then entirely removed from the solution using a rotary evaporator operating at 50oC. 200 mL of HCl (5%) is added and partitioned against 200 mL of chloroform (3 times) after the ethanol has been removed. The aqueous fraction is taken and basified by NH4OH (till pH=7). This aqueous phase is partitioned again against 200 mL of chloroform (3 times). Then the chloroform fraction is taken and evaporated by rotary evaporator at 40oC (Chart 2).
Total Phenolic Content (TPC): According to (Farhan, et al. [9]), the Folin-Ciocalteau reagent method has been utilized to estimate TPC. In a test tube, 1 mL of Folin-Ciocalteau reagent (1/10 dilution in water) was added to 100 L of each extract. 1 mL of Na2CO3 7% (w/v) has been added after 5 minutes. The mixture was incubated for 30 minutes at room temperature in the dark. Using a Gene Quant 1300 UV-Vis spectrophotometer, the absorbance of each sample's blue-colored solution was determined at 765 nm. In terms of dry weight of plant powders, the results were represented as mg of gallic acid equivalent (GAE) per g.
Total Phenol Content = GAE x V x D /m,
Where GAE is the gallic acid equivalence (mg/mL); V is the volume extract (mL), D is dilution factor and m is the weight (g) of the pure plant extract.
Biological Activities
Antioxidant Activity with 2,2-Diphenyl-1-Picrylhydrazyl (DPPH): The DPPH antioxidant test was utilized to determine the scavenging ability using the (Rammal, et al. [10]) A control was created by mixing 1 mL of DPPH with 1 mL of methanol at the same time as adding 1 mL of various doses of polyphenols and alkaloids (0.1, 0.2, 0.3, 0.4, and 0.5 mg/mL) to 1 mL of DPPH (0.15 mM in methanol). The reaction mixtures were thoroughly combined by hand, incubated at room temperature in the dark for 30 minutes, and the absorbance at 517 nm was measured using a Gene Quant 1300 UV-Vis spectrophotometer. Methanol served as the control and ascorbic acid as the positive control. Plant extracts' capacity to reduce DPPH was determined utilizing:
% Scavenging activity = [(Abs control − Abs sample)]/ (Abs control)] ×100
The Abs control is the absorbance of DPPH + alcohol; Abs sample is the absorbance of DPPH radical + sample. Also, three controls have been prepared.
Anticancer Activity
Using the human colon epithelial cells HT-29 and HCT-116, cell culture was done to investigate the antiproliferative activity of the powder's extracts from the examined plant. Next, the yellow tetrazolium MTT assay was used to measure the amount of cell growth inhibition. Cell culture was performed in 96-well plates, each containing 100 μL DMEM at 10.000 cells for HT-29 and 15.000 cells for HCT-116. The powder’s extracts were diluted with the DMEM culture medium in decreasing concentrations (200, 100, 50, 25 and 5 μg/mL) and were then added to the wells after pre-incubation for 24 h. The plates were then incubated under 5% CO2 and at a temperature of 37°C during 24, 48 and 72 hours respectively. After incubation, 10 μL of MTT solution were added per well and incubated for 3 hours at 37°C. Then a 100 μL solubilization solution was added to each well. Finally, the absorbance was measured with a spectrophotometer at 570 nm. This quantity is directly proportional to the number of cells with an intact membrane.
Antibacterial Activity Assay
Bacterial Strains: The strains used in this study were one Gram positive bacteria (Staphylococcus epidermidis CIP 444) and one Gram-negative strain (Escherichia coli ATCC 35218). The Gram-positive CIP 444 strain is a clinical strain that is isolated from an infected implanted device of a patient who is hospitalized in the Mignot Hospital of Versailles, France (Chokr et al. [11]). The identification and characterization of the properties of these strains were done and they were enclosed within the collection of microorganisms of Pasteur Institute in 2007 (Chokr, et al. [11-13]). The other strain is ATCC. The latter were kept at -80°C in glycerol stocks and used as needed. HIMEDIA (Mumbai, India) supplied the Mueller-Hinton broth (MHB), which was prepared and autoclaved as directed by the manufacturer before use.
MIC and MBC Assays: extracts of plant were tested for their corresponding Minimum Inhibitory Concentrations (MIC) and Minimum Bactericidal Concentrations (MBC) by broth microdilution assay, as recommended by the Clinical Laboratory and Standard Institute (CLSI). A concentration of 4.5 mg/ml of polyphenol purified extract and 3.5 mg/ml of alkaloid extract were prepared. In a 96-well plate (200 μl/well) (Greiner Bio-One, Essen, Germany), serial two-fold dilutions in MHB of the different extracts were done. The wells were inoculated with 5 × 105 bacteria/ml. After incubating the plates at 37°C for 24 hours, the MIC (which is defined as the lowest concentration that yielded no growth) was determined. In addition, the wells with no visible growth were placed on TSA in order to determine the MBC (which is defined as the lowest concentration which killed ≥99.9% of the initial inoculum). The Petri plates were incubated overnight at 37°C, and the MBC was determined.
Antibiofilm Activity
Dosage of Biofilm: coli biofilm formation was done according to a standard procedure. CFU/mL bacterial suspension contained in 100 μL of TSB medium supplemented with 0.25% glucose were inoculated in each well of 96-well microplate then incubated for 24 hours at 37°C. Their contents were then removed, and the biofilms formed in the wells were fixed by heating for 1 h at 80°C. The microplates were then kept cooling down at room temperature for 30 minutes then washed. Furthermore, 100 μL of crystal violet (0.1%) were added for each well for staining then washed after 5 minutes. Finally, 100 μL of ethanol was added and the optical density (OD) was measured at 570 nm using microplate reader (Tristar2 S, LB 942, Berthold, Germany).
Biofilm Prevention Activity and Determination of Minimal Biofilm Prevention Concentration (MBPC): One hundred microliters of TSB medium supplemented with 0.25% glucose and 100 μL of products were added to the first well of 96-well microplates and serial 1/2 dilution was done till 10th A diluted bacterial suspension was added as inoculum to each well to give a final concentration of CFU/mL. Wells lacking any product were used as positive control for biofilm formation. Wells without bacteria were used as negative control. The remaining steps of fixation, washing and staining were done as previously described and OD was measured. MBPC was defined as lowest concentration exhibiting highest significant prevention.
Statistical Analysis
The data of antibiofilm activity were expressed as the mean ± standard deviation (SD) of the optical density. Each experiment was repeated four times. Statistical analysis was done using Prism 5.0. Statistical differences were determined using One-way ANOVA followed by Dunnett post-test, when applicable, to compare results versus positive control. A p value of ≤ 0.05 was considered statistically significant.
TPC
The TPC estimated using the gallic acid standard curve (Figure 1) is 6.68 mg/g of dry powder.
Antioxidant/Scavenging Activity
The findings of the investigation on the antioxidant activity of crudely extracted polyphenols and alkaloids are shown in the graphs below. At 100 g/mL, they demonstrated a higher percentage of scavenging action (Figures 2 & 3). Moreover, the IC50 for the activity to scavenge DPPH radicals was 16 μg//mL and 22 μg//mL for polyphenols and alkaloids respectively.
Note: The Data are Mean Values from 3 Different Experiments. The Bars Represent Means with Standard Deviation.
*: Indicates a Statistically Significant Difference Compared to the Positive Control (p<0.05).
**: (p<0.01),
***: (p< 0.001). ns: non-Significant.
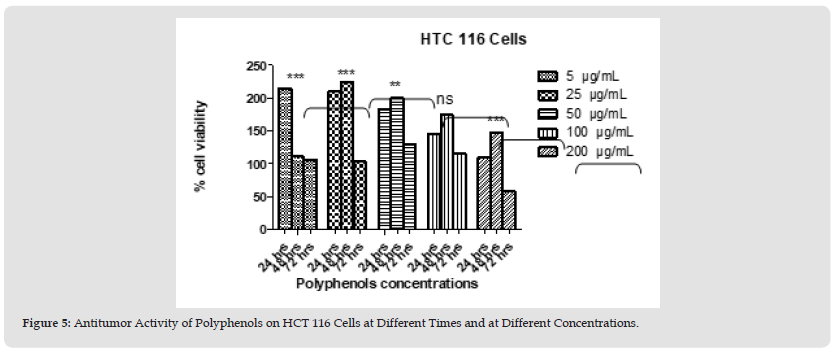
Figure 7 Figure 5: Antitumor Activity of Polyphenols on HCT 116 Cells at Different Times and at Different Concentrations.
Note: The Data are Mean Values from 3 Different Experiments. The Bars Represent Means with Standard Deviation.
*: Indicates a Statistically Significant Difference Compared to the Positive Control (p<0.05).
**: (p<0.01),
***: (p< 0.001). ns: non-significant.
Antiproliferative Activity
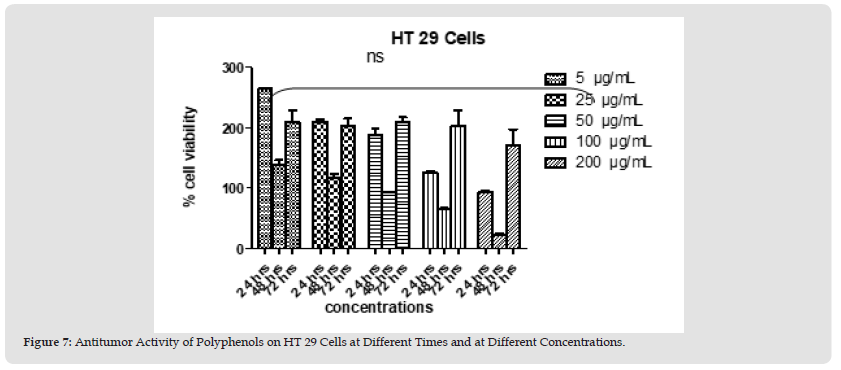
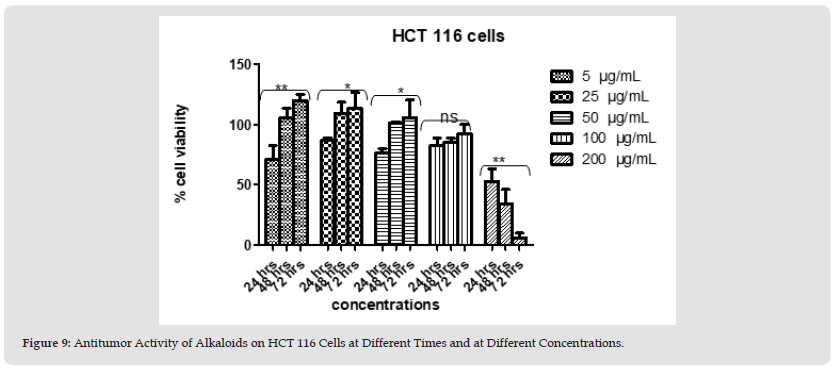
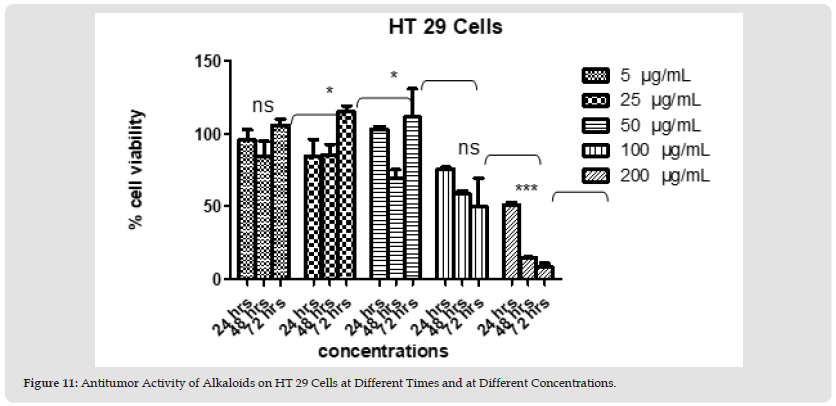
Polyphenols and alkaloids secondary metabolites were tested, in vitro, for their potential anti-proliferative capacity against HT-29 and HCT-116 cell lines. HCT-116 Cells were treated with a wide range of concentrations of polyphenols (25-200 μg/mL) to determine how this polyphenol affects the apoptotic process of tumoral cells. No antiproliferative activity was observed on HCT-116 cell lines at 24 hours & 48 hours. However, 42% of tumor cells died at the concentration value 200 μg/ml after 72 hours. After day 1, day 2, and day 3 (except concentration 200 μg/ml at day 3) we obtain a significant proliferation HCT 116 (Figures 4 & 5). Polyphenols also studied on HT-29 cells. The result is shown below in the (Figures 6 & 7): None of the doses had an inhibiting effect on the tumor cells after the addition of polyphenols for 24 hours. In contrast, they were proliferating in dose dependent manner (inversely proportional). With the exception of 5 and 25 μg/mL, which demonstrated HCT 116 cell growth after 48 hours after the introduction of the various concentrations, all concentrations indicated an increase in cytotoxicity. As concentration declined from 200 μg/mL to 50 μg/mL, respectively, the percentage of inhibition rose from 91.74% to 22.75%. Unexpectedly, for all doses, abrupt responses were noted after 72 hours. For all doses, there were either no outcomes at all or only a few. HCT-116 cells were also affected by different doses of Alkaloids (Figures 8 & 9). The effect was insignificant at dosages of 100, 50, 25 and 5 g/mL after 24 hours. At 200 g/mL, however, the percentage of inhibition rose to 47.3%. Following 48 hours of alkaloids being introduced to the cells, the inhibition was only achieved at the highest concentration of 200 g/mL, which had a significant inhibitory impact of 65.97%. At the lowest dose, there was no inhibitory impact after 72 hours. At doses of 100 g/mL and 200 g/mL, respectively, it was noted that the impact increased with the dose, from 7.7% to 93.74%. The results using the HT-29 cell line (Figures 10 & 11) demonstrated a strong inhibitory impact at the maximum dose (200 μg /mL), which was 49%. However, this percentage fell to 24.16 at 100 μg /mL as the dose was reduced. The inhibitory effect then dramatically reduced with the dose reduction until it was completely gone at 50 μg/mL. With 15.8% to 4.31% inhibition, the dosages of 25 μg/mL to 5 μg/mL, on the other hand, showed a very minor inhibitory impact. At the highest dose (200 μg/mL) after 48 hours, the percentage of inhibition was also the best, recording 85.34%. Then, at doses of 100 and 50 μg/mL, respectively, this% was reduced to 38.67% and 30.62%. It keeps going down proportionate to dose, reaching 14.81% at dose 25 μg/mL. After that, it marginally rises to 15.47% at 5 μg/mL. The inhibitory effect was at its peak (96%) at 200 μg/mL after 72 hours. At 100 μg/mL, a lower inhibitory effect (56%) is seen. However, as the dose was reduced, this% drastically shrank until it was completely absent at 50, 25, and 5 μg/mL.
Note: The Data are Mean Values from 3 Different Experiments. The Bars Represent Means with Standard Deviation.
*: Indicates a Statistically Significant Difference Compared to the Positive Antitumor Activity of Polyphenols on HT 29 Cells at Different Times. The Data are Mean Values from 3 Different Experiments. The Bars Represent Means with Standard Deviation.
*: Indicates A Statistically Significant Difference Compared to the Positive Control (p<0.05).
**: (p<0.01),
***: (p< 0.001). ns: non-significant.
Figure 9 Figure 7: Antitumor Activity of Polyphenols on HT 29 Cells at Different Times and at Different Concentrations.
Note: The Data are Mean Values from 3 Different Experiments. The Bars Represent Means with Standard Deviation.
*: Indicates a Statistically Significant Difference Compared to the Positive Control (p<0.05).
**: (p<0.01),
***: (p< 0.001). ns: non-significant.
Note: The Data are Mean Values from 3 Different Experiments. The Bars Represent Means with Standard Deviation.
*: Indicates a Statistically Significant Difference Compared to the Positive an Antitumor Activity of Alkaloids on hct 116 Cells at Different Times. The Data are Mean Values from 3 Different Experiments. The Bars Represent Means with Standard Deviation.
*: Indicates a Statistically Significant Difference Compared to the Positive Control (p<0.05).
**: (p<0.01),
***: (p< 0.001). ns: non-significant.
Figure 11 Figure 9: Antitumor Activity of Alkaloids on HCT 116 Cells at Different Times and at Different Concentrations.
Note: The Data are Mean Values from 3 Different Experiments. The Bars Represent Means with Standard Deviation.
*: Indicates a Statistically Significant Difference Compared to the Positive Control (p<0.05).
**: (p<0.01),
***: (p< 0.001). ns: non-significant.
Note: The Data are Mean Values from 3 Different Experiments. The Bars Represent Means with Standard Deviation.
*: Indicates a Statistically Significant Difference Compared to the Positive Antitumor Activity of Alkaloids on HCT 116 Cells at Different Times. The Data are Mean Values from 3 Different Experiments. The Bars Represent Means with Standard Deviation.
*: Indicates a Statistically Significant Difference Compared to the Positive Control (p<0.05).
**: (p<0.01),
***: (p< 0.001). ns: non-significant.
Figure 13 Figure 11: Antitumor Activity of Alkaloids on HT 29 Cells at Different Times and at Different Concentrations.
Note: The Data are Mean Values from 3 Different Experiments. The Bars Represent Means with Standard Deviation.
*: Indicates a Statistically Significant Difference Compared to the Positive Control (p<0.05).
**: (p<0.01),
***: (p< 0.001). ns: non-significant
Antibacterial Activity
MIC and MBC results are shown in the (Table 1) below. The term “bacteriostatic effect” was attributed to extracts having only MIC value. The term “bactericidal effect” was attributed to extracts having significant MBC value. Antibacterial nature of activities was classified according to ratio of MBC to MIC. Bacteriostatic activity was defined as a ratio of MBC to MIC >4 while bactericidal activity as a ratio of MBC to MIC <4. The nature of activity of extracts lacking a MBC value was considered as not determined. The sensitivity of tested Gram-positive and Gram-negative bacteria to different extracts was variable. The antibacterial activity of the polyphenols (MIC=1.125mg/ml) exceeded that of alkaloids (MIC=1.75 mg/mL) against E. coli and S. epidirmedis (Table 2-3).
Table 1: In vitro Pharmacodynamics Parameters of Bacterial Growth Inhibition and Bactericidal Activity of Polyphenols and Alkaloids on S. Epidermidis and E. Coli Using Quantitative Microdilution Method.
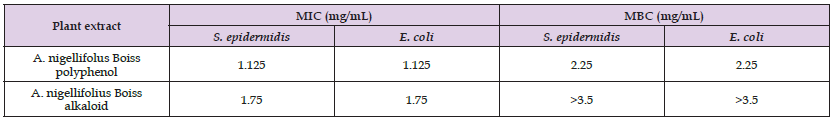
Note: N/A: Not Applicable, ND: Not Determined.
Antibiofilm Activity
Prevention of Biofilm Formation: The results of preliminary tests of antibiofilm prevention activity by polyphenols and alkaloids are shown in graphs below. nigellifolius extracts variously modified biofilm formation of E. coli. The prevention percentage is calculated as following:

The minimal biofilm prevention concentration (MBPC) corresponded to the lowest concentration capable with the highest percentage of preventing biofilm formation.
Biofilm Prevention by Polyphenols: A significant (p<0.01) decrease in biofilm formation was observed when E. coli grown in the presence of the polyphenols. Polyphenols exhibit 74.3% of biofilm prevention with MPBC equal to 48.4 mg/mL (Figure 12).
Biofilm Prevention by Alkaloids: A significant (p<0.01) decrease in biofilm formation was observed when E. coli grown in the presence of the alkaloids. Alkaloid extract prevented 74.4%, 63%, 34 %, and 20% at concentration 43.7 mg/mL, 21.8 mg/mL, 10.9 mg/mL, and 5.4 mg/mL respictively with a MBPC value of 43.7 mg/mL (Figure 13).
Note: Negative Control is MHB + 0.25% Glucose Media. The Data are Mean Values from Two Independent Experiments with Two Wells in each,
*: Indicates a Statistically Significant Difference Compared to the Positive Control (P<0.05),

Note: Negative Control is MHB + 0.25% Glucose Media. The Data are Mean Values from Two Independent Experiments with Two Wells in Each,
*: Indicates a Statistically Significant Difference Compared to The Positive Control (P<0.05), : Indicates MBPC.
The diets of humans and other animals have always included a range of phytochemicals, which are secondary metabolites of plants. Although a large number of these phytochemicals have traditionally been thought to have little utility in plants (although this perception is shifting), it has regularly been demonstrated that when consumed by animals, they have negative consequences. Alkaloids, carbohydrates, and non-protein amino acids as well as a wide range of polyphenolic and terpenoid compounds, as well as hydrophilic and hydrophobic compounds, can be classified as phytochemicals (Acamovic, et al. [14]). Phenolic compounds, ubiquitous in plants, are an essential part of the human diet and are of considerable interest due to their antioxidant properties and potential beneficial health effects. They have been shown to suppress plasma platelet aggregation, cyclooxygenase activity, histamine release, and to have antibacterial, antiviral, anti-inflammatory, and antiallergenic properties. They also lower the risk of cancer, heart disease, and diabetes. The antioxidant property of phenolics contributes to the advantages against several of these illnesses. Thus, it's critical to measure, identify, and assess their anti-inflammatory, anti-proliferative, and antibacterial actions (Shahidi, et al. [15]). The polyphenols isolated from the Lebanese A. negillifolius plant were tested for their antioxidant activity in vitro in the current study.
The data obtained demonstrate that the presence of polyphenols causes radical chromogens to vanish, and the activity in doing so is determined by the absence of color or absorbance readings produced from a particular UV spectrum. The efficacy of crude polyphenols to scavenge DPPH improved with higher polyphenol concentrations. The scavenging rate was 35% and 89% at concentrations of 5 and 100 μg/mL, respectively. The DPPH radical-scavenging activity had an IC50 of 16 μg/mL. As a result, the outcomes of this assay demonstrated that polyphenols have strong antioxidant activity. Free radicals have been shown to significantly impact cardiovascular illnesses, aging, cancer, and inflammatory diseases. They also cause oxidative damage to biomolecules (Jimenez, et al. [16]). Several studies have demonstrated the potent in vitro antioxidant effects of polyphenols (Jurinjak Tusek, et al. [17]). Likewise, the results of the current investigation demonstrated that polyphenols were the primary antioxidants that interact with free radicals and exhibited significant free-radical repression. The studies revealed that many polyphenol-rich plant extracts from food and herbal medicine, as well as isolated polyphenols administered alone or in combination, can regulate cell apoptosis primarily through intrinsic and extrinsic mechanisms of action in in vitro conditions. This is in reference to the antiproliferative activity of extracted polyphenols and alkaloids (Curti, et al. [18]). The use of polyphenols in the treatment of cancer should thus be thoroughly researched in light of these encouraging results (Curti, et al. [18]). The apoptotic effects of polyphenols were discovered to be selective.
According to our findings, polyphenols have been shown to have pro-apoptotic effects on malignant cells via regulating pathways involved in cell growth and death in a dose-dependent manner. HCT-116 cells were treated with a wide range of concentrations of polyphenols (25-200 μg/mL) to determine how this polyphenol affects the apoptotic process of tumoral cells. It has been demonstrated that the best method for preventing the spread of these cells is to apply 200 g/mL, which will not produce an immediate result but will become effective after 72 hours. Concerning the antiproliferative activity of polyphenols on HT-29 cells, it was showed that the effect of different concentrations applied of polyphenols varied against HT-29 cells. The IC50 was detected when cells were treated with polyphenols concentration equal to 100 μg/mL at 48 hours. It is clear that polyphenols have satisfactory results on HT-29 cells. This effect was noticed after 48 hours from the beginning of the experiment with results of 91.74% for 200 μg/mL. However, no inhibitory effect observed after 72 hours of being in contact. This result can be explained by the disappearance of polyphenols. Those polyphenols may be either transformed into other molecules that exert no effect on HT-29 cells or they show resistance against these phytochemicals. Furthermore, results showed that inhibitory effect exerted by the polyphenols on HT-29 cells was greater than that exerted on HCT-116 cells. This difference in effect of polyphenols between HCT-116 and HT-29 may be due to the presence of receptors specific to polyphenols on HT- 29 cells in great amount than that present on HCT-116 cells.
At certain concentrations as shown in result part, there was an increase in cell proliferation. This can be explained by presence of other molecules that trigger cell cycle. In this case, the % of proliferation was decreasing as the concentration of polyphenol was increasing. This result may be due to dominant effect of polyphenols over those molecules. Other hypothesis is that these molecules exert their effect at low concentrations. Growth inhibitory effect and induction of apoptosis by alkaloids were investigated using HCT-116 and HT-29 cell lines. Regarding HCT-116 cells, there were also reasonable effects for alkaloids. The % inhibition effect increased with the increase of doses having best results at 200μg/mL after 24 hours. This shows that alkaloids have an effect on these cells at high doses. So, we obtain satisfactory results concerning the antiproliferative activity of purified alkaloids on HT-29 cells at high concentrations. On the other hand, negligible effects were recorded for low doses, while enormous results were recorded for the 200μg/mL dose starting from day1 (48%), and the effect reached its maximum after 72 hours (96%). This shows that alkaloids are effective only at high doses (200μg/mL). Concerning studying the effects of secondary metabolites on microorganism, S. epidermidis and E. coli were chosen. Amongst the various species studies, S. epidermidis was found as the most recurrently isolated specie and the most widespread bacterium in human skin. This is due to the fact that S. epidermidis’ infections are generally obtained in hospitals due to contamination of surgical incises from the patients themselves or from the hospital personnel.
The infection with E. coli is one of the most serious impediments in burnt patients superinfected. Polyphenols and alkaloids tested for their antibacterial potencies showed an antibacterial potency against different strains using microdilution method. Obtained results showed significant antibacterial activity of these products on E. coli and S. epidermidis at low concentrations. In phenolics, multiple mechanisms of antibacterial activity have been described: they interact with bacterial proteins and cell wall structures, they may cause damage to cytoplasmic membranes, reduce membrane fluidity, inhibit nucleic acid synthesis, cell wall synthesis, or energy metabolism (Daglia, [19]). The MIC of the Tea Polyphenols was 6.25 mg/mL on E. coli (Yin, et al. [20]). In our research we aimed to study the antibacterial and the antibiofilm potency of A. negillifolius Boiss secondary metabolites against E. coli. biofilms formed by pathogenic E. coli strains can pose serious problems to human health such as prostatitis, biliary tract infections, and urinary catheter cystitis (Ren, et al. [21]). Antibiofilm activity research on plant phenolics has revealed, besides their destructive activity on bacteria, also “softer” activities leading to biofilm suppression by affecting the bacterial regulatory mechanisms such as quorum sensing or other global regulator systems, without an effect on bacterial grow (Silva, et al. [22]). Recently, there has been a tremendous increase in biofilm research, most of it with the ultimate aims of biofilm prevention, control, or eradication (Labbate, et al. [23]).
The prevention of biofilm process could be either due to inhibiting bacterial growth mechanism or inhibiting the biofilm formation mechanism or both. Polyphenols and alkaloids inhibited biofilm formation only at concentrations higher than MIC. Regardless the different conditions in the two experiments (experiment of antibacterial and experiment of antibiofilm), the results shows that their inhibition of biofilm is due to their capability of inhibition of bacterial growth only. Several phytochemicals had been shown to inhibit biofilm formation including alkaloids, fatty acids, phenols and terpenoid (Silva, et al. [22]). Among all extracts that exhibited strong biofilm prevention potency (>90%), phenols and terpenoids were the common phytochemical. Phenols and terpenoids are well known to prevent biofilm formation. Zhang, et al. (2-14) studied the antibiofilm activity of polyphenolic extract from Rosa rugosa tea (RTP). The products were capable of preventing biofilm formation of different strains. For example, at the concentration of 640 g/mL, RTP showed a maximum of 67.02% and 72.90% reduction in biofilm biomass of E. coli and P. aeruginosa.
The objectives of our present work were to extract and purify polyphenols and alkaloids from leaves and stems of fresh Lebanese Anacyclus nigellifolius Boiss and to evaluate its antioxidant, anti-tumor, and antibacterial activities. The information suggested that polyphenols and the physiologically active chemicals they contain might be sources of fresh antioxidants. Also, our findings showed that A. nigellifolius had antibacterial effects on planktonic and biofilm-living E. coli and S. epidermidis as well as an antiproliferative effect on the viability of HCT-116 and HT-29 cells. However, more in vivo and clinical research is required to show that these substances are effective as treatments for a variety of free radical-related disorders, including cancer.
Using an HPLC/MS or similar analytical method, the chemical composition of these extracted polyphenols and alkaloids will be identified in a subsequent investigation.


