Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Özlem Gündoğdu1, Serkan Şen2 and Nurhan Kishali3*
Received:March 03, 2023; Published:March 15, 2023
*Corresponding author: Nurhan Kishali, Atatürk University, Science Faculty, Department of Science, 25240-Erzurum, Turkey
DOI: 10.26717/BJSTR.2023.49.007775
A new and convenient synthesis for pyrrolidine derivatives has been developed starting from 3a,4,7,7atetrahydroisobenzofuran- 1,3-dione. 3a,4,7,7a-tetrahydroisobenzo furan-1,3-dione was reacted with amines to give N-ethyl and N-phenyl isoindole derivatives. These amine compounds are reduced to bicyclic pyrrolidine derivatives with LiAlH4. Pyrrolidine derivatives absorption and fluorescence spectra were recorded with different concentrations and solubility systems. One of the derivatives exhibited the highest fluorescence quantum efficiency in ethanol and the other in DCM.
Scientists have realized that alkaloids are vital to biology, medicine, and chemistry. According to biologists, alkaloids are pure and extraordinarily natural products. For pharmacists, alkaloids are nitrogenous substances of plant or animal origin, generally of complex structure and high molecular mass. Chemists have defined alkaloids with naturally occurring complex nitrogenous heterocyclic structures and strong physiological activity, basic, generally toxic, as organic compounds [1-3]. Most of them have therapeutic properties as medicines. The earliest known about alkaloids is the therapeutic use of extracts prepared from the Ephedra Chinensis plant in BC 27004. The beginning of alkaloid chemistry in the modern sense is considered to be the isolation of morphine from the opium plant (Papaver Somniferum) in 1804 by Friedrich Wilhelm Adam Sertürner. Then, P. J. Pelletier and J. B. Caventou isolated some other alkaloids such as Brucine, Caffeine, and Veratridine between 1817 and 1821 [1] (ss 1). Although it has been known for a long time, developments in the field of spectroscopy have led to a rapid increase in information about isolated alkaloids. With the discovery of compounds such as vincristine isolated from the Catharanthus Roseus plant, which is one of the important drugs used in cancer treatment, studies for the development of alkaloids are gaining momentum (Figure 2). The number of alkaloids whose structures are illuminated in various sources is stated to be 6000-10000 [3]. Alkaloids are classified according to the properties of the heterocyclic ring they have.
In the ring system classification, the first member is Pyrrolidine, which contains nitrogen atoms in the five-membered ring. Pyrrolidine ring is frequently seen in the structure of natural compounds, especially amino acids and proteins. Natural and bioactive compounds such as vitamin B12 [4], nicotine [5], nornicotine [6], hygrine, hygroline, and cuscohygrine [7] are some of the pyrrolidine alkaloids [4-6] (Figure 3). Many compounds containing pyrrolidine ring have different bioactive properties such as antitumor [8], analgesic [9], anesthetic [10], antifungal [11], antibacterial [12], insecticidal [13], anthelmintic [13], antileukemic [14], anticonvulsant [14], and antidepressant effects [15]. Some pyrrolidine derivatives have also been shown to have an enzyme inhibitory effect [16-18]. In addition, pyrrolidine derivatives that show antiviral activity have also been determined [19-22]. For example, 2- (1-adamantyl) pyrrolidine has been found to have antiviral activity on influenza A H2N2 [20]. The increasing use of heterocyclic compounds such as pyrrolidine and their derivatives as building blocks of a large number of materials and biologically active molecules has made their synthesis important. It is also important to determine the spectroscopic properties of the synthesized compounds. Here, we describe an efficient synthesis of pyrrolidine derivatives 1 and 2 using tetrahydroisobenzofuran-1,3- dione (Scheme 1) and their fluorescent properties.
Note: Reagents and conditions:
(a) Xylene, reflux, 5 h;
(b) Toluene, reflux, 36 h;
(c) LiAlH4 (1.0 M solution in THF), THF, 70 °C, 16 h.
Synthesis
To synthesize pyrrolidine derivatives, firstly tetrahydroisobenzofuran-1,3-dione (1) 3-sulfolene and maleic anhydride were synthesized by intramolecular [4 + 2] cycloaddition reaction as we did in our previous studies [23]. It was then converted into tetrahydroisobenzofuran-1,3-dione ethylamine and anilinerelated imide derivatives 2 and 3. Then the N-ethyl and N-phenyl isoindole derivatives were subjected to LiAlH4 reduction in THF. With this procedure, we offer a method for the syntheses of bicyclic pyrrolidine derivatives 4 and 5 under easily reproducible reaction conditions. The structures of the products formed in the reaction were determined by NMR spectroscopy techniques. The structure of 3 was assigned based on its 1H-NMR spectra, which revealed the presence of three different five CH2 groups, and one methyl group. The most remarkable features deduced from the 1H NMR spectrum of 4 was the presence of the aromatic H atoms and the incorporation of the amino functionality in the molecule. The 13C NMR spectrum exhibited signals of three aliphatic N linkage C atoms in 3 and 4.
Absorption and Fluorescence Spectra Properties
Absorption and fluorescent spectra of pyrrolidine derivatives 4 and 5 were recorded between 5x10-5 and 5x10-2 M. It was observed that peak intensities increased linearly as the concentration increased in the absorption spectra. Peak positions did not differ significantly in different solvent media: ethanol (EtOH), acetonitrile (ACN), or dichloromethane (DCM), and the band at near UV region is attributed to characteristics of those pyrrolidine derivatives [24,25]. These values are given in Table 1. The emission maxima of pyrrolidine derivatives in EtOH, ACN, and DCM are given in Table 1. Both pyrrolidine derivatives (4 and 5) exhibit high fluorescence at the blue region of the electromagnetic spectrum. Normalized fluorescence spectra of these compounds are given in Figure 4 and Figure 5. As seen in Figure 5, phenyl substituted derivative shows hypsochromic shift in ethanol as a polar media, which can be explained by H bond interactions between the molecule and the solvent [26-28]. Fluorescence quantum yields compiled in Table 1 vary upon the substituent and solvent effect. The highest fluorescence quantum yields were calculated in ethanol for the ethyl substituted derivative and dichloromethane for the phenyl substituted derivative. However, for compounds 4 and 5 quantum yields are high as well in other solvents.
Absorption and Fluorescence Spectra Properties
Absorption and fluorescent spectra of pyrrolidine derivatives 4 and 5 were recorded between 5x10-5 and 5x10-2 M. It was observed that peak intensities increased linearly as the concentration increased in the absorption spectra. Peak positions did not differ significantly in different solvent media: ethanol (EtOH), acetonitrile (ACN), or dichloromethane (DCM), and the band at near UV region is attributed to characteristics of those pyrrolidine derivatives [24,25]. These values are given in Table 1. The emission maxima of pyrrolidine derivatives in EtOH, ACN, and DCM are given in Table 1. Both pyrrolidine derivatives (4 and 5) exhibit high fluorescence at the blue region of the electromagnetic spectrum. Normalized fluorescence spectra of these compounds are given in Figure 4 and Figure 5. As seen in Figure 5, phenyl substituted derivative shows hypsochromic shift in ethanol as a polar media, which can be explained by H bond interactions between the molecule and the solvent [26-28]. Fluorescence quantum yields compiled in Table 1 vary upon the substituent and solvent effect. The highest fluorescence quantum yields were calculated in ethanol for the ethyl substituted derivative and dichloromethane for the phenyl substituted derivative. However, for compounds 4 and 5 quantum yields are high as well in other solvents.
General
All reagents used were commercially available unless otherwise specified and all solvents were distilled before use. UV/ VIS Absorption and fluorescence spectra: Perkin Elmer Lambda 35 UV/VIS spectrophotometer and a Shimadzu RF-5301PC spectrofluorophotometer, respectively. Fluorescence and absorption measurements of all bicyclic pyrrolidine derivatives were performed at r.t. in DMC, ACN, and EtOH. For the steady-state fluorescence measurements, all the samples were excited at 350, and fluorescence intensities were recorded between 360 and 700 nm. Fluorescence quantum yield ( Φf ) for pyrrolidine derivatives was calculated by using the Parker-Rees equation [29]. Quinine sulfate in a 0.5 M H2SO4 solution was used as the reference. 1H and 13C NMR spectra: Varian 400 and Bruker 400 instruments; in CDCl3. Elemental analysis: Leco CHNS- 932 instruments.
General Procedure for the Preparation of 4 and 5
Compounds 2 and 3 were synthesized as described in 24 lit. Then to convert to 4 and 5, a magnetically stirred suspension of LiAlH4 (6 mmol) in dry THF (20 mL) was added to the solution of 4 or 5 in THF (1g, 1 mmol) at 0 0C under N2 atm. Then the reaction mixture was stirred overnight at reflux. During this time, the reaction was monitored by TLC. The solvent was evaporated. EtOAc (20 mL) was added, and the mixture was washed with saturated ice water (30 mL). The solid was filtered off and extracted with EtOAc (3 × 20 mL). The combined organic phase dried over Na2SO4. The product mixture was purified by silica gel column chromatography. The solid was filtered off and extracted with EtOAc (3 × 20 mL). The combined organic phase dried over Na2SO4. The product mixture was purified by silica gel column chromatography.
1H-NMR (400 MHz, CDCl3) 4: δ 5.74 – 5.72 (m, 2H), 2.93 – 2.89 (m, 2H), 2.44 – 2.38 (m, 2H), 2.36 – 2.28 (m, 2H), 2.16 – 2.06 (m, 4H), 1.81 – 1.76 (m, 2H), 1.03-0.09 (t, 3H). 13C-NMR (100 MHz, CDCl3) 4: δ 127.67, 60.57, 50.73, 35.60, 26.56, 13.99. Anal. calc. for C10H17N (151,14) C, 79.41; H, 11.33; N, 9.26, found: C, 79.75; H, 11.33; N, 9.60.
1H-NMR (400 MHz, CDCl3) 5: δ 7.21 (tdd, J = 9.5, 3.2, 1.8 Hz, 2H), 6.63 (t, J = 7.3 Hz, 1H), 6.51 (d, J = 7.8 Hz, 2H), 5.67 (s, 2H), 3.39 (dd, J = 8.7, 6.4 Hz, 2H), 3.10 (dd, J = 8.8, 5.3 Hz, 2H), 2.50 – 2.39 (m, 2H), 2.35 – 2.22 (m, 2H), 2.06 – 1.90 (m, 2H). 13C-NMR (100 MHz, CDCl3) 5: δ 148.19, 129.13, 124.69, 115.00, 111.04, 52.90, 34.28, 25.23. Anal. calc. for C14H17N (199,30) C, 84.37; H, 8.60; N, 7.03, found: C, 84.24; H, 8.88; N, 6.87.
The fluorescence and UV-Vis absorption spectra of samples were recorded with a Shimadzu model RF 5301PC Spectrofluorophotometer and Perkin Elmer Lambda 35 UV/Vis Spectrophotometer with quartz cuvettes, 1.0 cm optical path length, respectively. Fluorescence quantum yield values ( Φf ) of these samples from corrected fluorescence spectra were calculated by using the equation given below [30]. Where D is the integrated area under the corrected fluorescence spectrum and n is the refractive index of the solution. The subscripts s and r refer to the sample and the reference, respectively. In addition, ODs and ODr are the optical densities for the sample and the reference at the excitation wavelength, respectively. For the determination of relative quantum yield values, Quinine sulfate in 0.5 M H2SO4 solution (ɸf =0.546) was used as a reference [31,32].
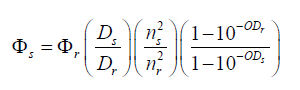
In this study, pyrrolidine derivatives were synthesized by a simple and effective method. These compounds, which have cyclic amine groups, are very important as starting materials for natural product chemistry. These compounds’ optical properties were investigated by UV-Vis absorption spectroscopy. The optical parameters were compared with each other. The UV-Vis spectra of these compounds were recorded in EtOH, ACN, and DCM. The absorption and fluorescence spectra of the compounds were recorded at concentrations between 5x10-5 and 5x10-2 M. It was observed that as the concentration increased, the absorption peak intensities increased linearly. The positions of the peaks in different solvent environments (ethanol (EtOH), acetonitrile (ACN), or dichloromethane (DCM)) did not differ significantly. Also, band formation was observed in the near UV region. Both cases are attributed to the structural properties of the pyrrolidine derivatives. In the blue region of the electromagnetic spectrum, both pyrrolidine derivatives exhibited high fluorescence. The pyrrolidine phenyl derivative showed a hypsochromic shift in ethanol. The reason for this can be shown in ethanol as a polar medium, the interactions between the molecule and the solvent.


