Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Moschos G*, Theodoridou A, Stylianidou S, Capizzello A and Plataniotis G A
Received:February 08, 2023; Published:March 14, 2023
*Corresponding author: Moschos Georgios, University of Thessaloniki, Radiotherapy Department, AHEPA Hospital 1St Kyriakidi str, Thessaloniki, Greece
DOI: 10.26717/BJSTR.2023.49.007772
Introduction: We present our current experience regarding radiotherapy (RT) of early glottic cancer,
and we investigate the possibility for further improvement with hypofractionation.
Methods: Patients treated between May 2016 and October 2021 with definitive radiotherapy for T1-2
N0 squamous-cell carcinoma of the glottis were reviewed retrospectively. The probabilities of local
control, ultimate local control (after salvage surgery), cause specific survival (CSS) and overall survival
(OS) are presented.
Results: We report on 57 treated patients, with median age of 68 years (49 to 88 years). The median
follow-up time was 46 (12 to 74) months. 20 (35.1%), 13 (22.8%) and 24 (42.1 %) of patients had
T1a, T1b and T2 disease respectively. Five-year actuarial local control rates were as follows: T1 90.9
%, T2 79.2 % (p=0.205) and overall 86%. Local control rates after salvage surgery were: T1 97 % and
T2% 95.8% (p=0.887) and overall 96.5 %. Five-year disease specific survival and overall survival were
96.5% and 86% respectively. There were no significant associations with T-stage, overall treatment
time, histological grade with local control on univariate analysis. Only 2 patients (3.5 %) required
short-term parenteral nutrition during radiotherapy. One patient required a tracheostomy due to a
non-functioning larynx one year post radiotherapy. Second primary cancers developed in 17.5 % of
patients.
Conclusion: Our results show that there might be room for improvement for both T1 and T2, but
especially T2-staged tumors. A reasonable way to investigate, is the employment of hypofractionation
(HFX) RT.
Keywords: Early-Stage Glottic Cancer; Radical Radiotherapy; Hypofractionation
Abbreviations: CSS: Cause Specific Survival; OS: Overall Survival; HFX: Hypofractionation; CT: Computerized Tomography; VMAT: Volumetric Modulated Arc Therapy; CTCAE: Common Terminology Criteria for Adverse Events
Laryngeal cancer accounts for 0.7 % of all new cancer cases and 75 % of laryngeal carcinomas arise from the vocal cords (glottis) [1]. Glottic carcinoma is usually diagnosed at earlier stages with generally low risk of lymphatic spread [1]. Disease control and larynx preservation are the two main aims of treatment with radiotherapy (RT). Radiotherapy and transoral laser resection have comparable disease control rates, as reported from retrospective rather than randomized trials. The 5-year local control rates following RT, ranges from 80 to 95% for T1 and 65–85% for the T2 larynx cancer [2-5]. A wide range of radiotherapy dose-fractionation schedules have been employed for the treatment of early glottic carcinoma [6-9] although short fractionation RT schedules are not widely accepted so far [10]. This retrospective study and review will audit the outcomes in a series of patients with early glottic cancer treated at a single center by definitive RT.
Patients treated between May 2016 and October 2021 with definitive radiotherapy for early glottic cancer were retrospectively identified from radiotherapy databases and patient notes. Inclusion criteria were biopsy proven invasive squamous cell carcinoma of the glottis, T1 or T2 N0 disease and definitive radiotherapy; patients with prior therapeutic minor surgery (e.g., laser stripping, cordectomy) were also included. Patients with a follow-up period shorter than 12 months from the start of RT were excluded.
Radiotherapy Technique
Patients underwent simulation computerized tomography (CT) scan in supine position, and they were immobilized on treatment position by a thermoplastic mask. The patients were instructed not to swallow during CT simulation and treatment. For the majority of patients RT was delivered using 3D technique via lateral parallel opposed or anterior oblique paired fields. Eight patients were treated with volumetric modulated arc therapy (VMAT) technique. Standard field borders were:
1. Superior: mid thyroid notch
2. Inferior: bottom of cricoid cartilage
3. Anterior: 1 cm anterior to skin of neck
4. Posterior: anterior to vertebral body.
Neck radiotherapy was not used for any patient. Treatment was planned with 6MV photons and prescribed in accordance with the ICRU 50 recommenda¬tions. Treatment delivery was with a 6MV linear accelerator with 1 cm multileaf collimators. Some patients were treated with 2.0 Gy per fraction to a total dose of 64-70 Gy and the rest were treated with 2.1-2.3 Gy per fraction to a total dose of 61.6-66 Gy.
Follow Up
Patients were regularly evaluated by performing laryngoscopy during treatment. Thereafter, patients were followed up at 3, 6 months and 1 year after RT completion. In the second year, the patients were followed up at 6-monthly intervals. Post-treatment imaging was carried out if clinically indicated. Acute and late toxicities were graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 5. Toxicity that occurred during the RT course or less than 3 months after RT completion was categorized as acute toxicity and later events were categorized as late toxicity.
Statistical Analysis
Statistical analysis was performed using SPSS version 16 (IBM, USA). Survival and recurrence outcomes were calculated from the last date of radiotherapy treatment. The following endpoints were used for assessment: local control, ultimate local control (including salvage treatment), cause specific survival (CSS) overall survival (OS), and were analysed using Kaplan–Meier product limit curves. Univariate Cox proportional hazards regression analysis was performed for the following factors: age, gender, histological differentiation, T stage (T1 versus T2), fractionation type (conventionally fractionated versus HFX), overall treatment time (more versus less than 42 days) and prior laser therapy.
Patient and Treatment Characteristics
57 patients were identified in this retrospective study. Patient and treatment characteristics are summarised in (Table 1). All patients had biopsy proven squamous cell carcinoma and had undergone a staging CT/MRI of larynx. Eight patients had undergone prior laser treatment for early glottic carcinoma; and radiotherapy was delivered to these patients according to evidence of residual disease following laser treatment. Median duration of radiotherapy was 42 days (range 32 to 64 days). Treatment duration was greater than 50 days in 3 patients; reason for delay was patients’ compliance for two patients and not documented for one other patient.
Disease and Survival Outcomes
56/57 of patients had a complete clinical response to treatment; the patient with persistent disease underwent a salvage total laryngectomy. Five-year actuarial local control rates were as follows: overall 86 %, T1 90.9% (T1a 95%, T1b 84.6%) and T2 79.2% (p=0.205). Ultimate local control rates (including successful salvage treatment) were: overall 96.5 %, T1 97 % and T2% 95.8% (p=0.887). A total of 7/57 patients experienced disease recurrence after the initial complete clinical response; 8 patients experienced local recurrence only after a median of 12 months (3 to 32 months) post radiotherapy. Of local recurrences, 7/8 were within the larynx. One patient developed regional neck recurrence in combination with recurrence at the primary subsite. A total of 8 patients underwent salvage surgery and 6 of these patients are disease free and alive. 5/8 patients underwent total laryngectomy with or without neck dissection, 2 patients underwent hemilaryngectomy and 1 patient underwent laser cordectomy. Given the retrospective nature of this review, it was not possible to evaluate consistent details regarding surgical complications. The patterns of recurrence are summarised in (Table 2). A total of 31/57 patients completed radiotherapy within 42 days, and 6/8 patients that experienced recurrence had overall treatment time > 42 days, although univariate analysis did not reveal any significant association between local control and overall treatment time (more versus less than 42 days, p=0.06). Univariate analysis of covariates did not reveal any significant association with local control and overall survival (all p >0.05). (Figure 1) illustrates 5-year local control in different stages and overall treatment time (OTT) groups. Five-year cancer specific survival (CSS) and overall survival (OS) were 96.5% and 86% respectively; a total of 8 deaths occurred during follow up. The cause of death was recurrent larynx cancer in 2 and death with other reasons in 6 patients.
Second Malignancy
Ten (17.5 %) patients developed second cancers during followup. The most common site of a second primary tumour was lung cancer, occurring in 4 (40%) patients and colon carcinoma in 2 (20%) patients and four patients developed other neoplasms.
Toxicity Outcomes
Details of acute and late toxicities are listed in (Table 3). Documented acute hoarseness, mucositis and radiation dermatitis were mainly of grade 1-2. Two patients with grade 3 mucositis (3.5%) required temporary enteral nutrition and two patients developed grade 3 acute hoarseness (3.5%) treated with low doses of dexamethasone. No grade 4-5 toxicity was recorded. As regards late toxicities, late mucositis and radiation dermatitis of grade 1-2 were recorded in 5 (8.7%) and 7 (12.2%) respectively. Late hoarseness of grade 1-2 was recorded in 27 patients (43.8%). There was only one patient (1.7%) with grade 3 late toxicity (hoarseness) and no evidence of disease recurrence requiring a tracheostomy one year post radiotherapy. No patients required long term enteral feeding.
We report our recent experience on RT of early stage glottic carcinoma acknowledging the limitations of a rather low number of patients, the retrospective character of the study and the rather short follow up time for some of the patients. However, our results are comparable with the literature regarding tumour control and post-radiation toxicity. Chera, et al. [11]. reviewed eleven large studies documenting 5-year local control rates of patients with early glottic cancer treated by definitive radiotherapy and using a mix of conventional and HFX schedules. Those studies used the 1998 TNM classification (subdividing T2 disease into T2a and T2b, cord mobility) and 5-year local control rates for T1a, T1b, T2a and T2b disease were 94%, 93%, 80% and 70% respectively [11]. Compared to our series, 5-year local control rates were generally comparable (apart from probably T1b, our control rate is lower) at 95%, 84.6% and 79.2% for T1a, T1b and T2 disease. Although our disease control rates are not dramatically different from those reported from other studies, it seems that for T1b and T2-staged tumours there is some room for improvement. Keeping all technical parameters of RT the same, we believe that our RT outcomes can be definitely improved by only adapting the routine use of HFX for the treatment of early glottic cancer. Moreover, this is expected to reduce the OTT, the number of RT fractions and therefore save RT resources and in addition reduce the number of patients’ visits to the hospital especially in the era of the ongoing covid-19 pandemic. We thoroughly reviewed the relevant literature on laryngeal cancer HFX RT and found quite convincing evidence in favour of HFX in this type of cancer. As it is not possible, to present our extensive literature review in this paper, we picked up a large retrospective study involving 10.212 patients (taken from the National Cancer Data Base registry data for patients diagnosed from 2004 to 2013) published in the Journal of NCI, which unequivocally favours HFX RT especially for T2 glottic cancers [12].
Radiobiological Considerations
A conventionally fractionated course of radiotherapy for squamous-cell carcinoma has been generally 66-70 Gy in 6.5-7 weeks, one fraction per day, of 1.8-2 Gy. However, there have been many attempts to improve radiotherapy outcomes over the years, in multiple clinical trials in which the alteration of all the parameters of dose-time-fractionation such as the number of RT fractions, the overall treatment time and the total dose (TD) was tried. A schedule involving two fractions per day of, say 1.2 Gy per fraction, 6 hours apart is called “hyperfractionated” and it results in a moderate shortening of the OTT (compared to the standard schedules of 2Gy/ day) as the daily dose is 2.4 Gy. Shorter OTT reduces the amount of repopulation in tumors and may increase disease control rates. We note here that in our clinical material we found a non-statistically significant trend (p=0.06) of a better local control rate in patients with OTT shorter than 42 days. In addition, low dose per fraction (1.2 Gy) spares late reacting normal tissues, as the Linear-Quadratic model also predicts and consequently allows the prescription of higher TDs which may increase TCP [13-14]. Other RT schedules such as CHART (Continuous Hyperfractiona¬ted Accelerated RT) used in the UK for more advanced tumors, deliver three-fractions per day and complete RT in just 12 days [15]. This fractionation variant is known as “accelerated hyperfractionation”.
In the last 10 years the technical developments have remarkably increased the precision in RT treatment planning and RT delivery (Intensity Modulated RT-IMRT, Image Guided RT-IGRT, Tomotherapy) and have allowed radiation oncologists to use another type of altered fractionation called “hypofractiona¬tion” which employs daily dose (per fraction) of higher than 2 Gy, for instance 2.2-2.75 Gy or even higher [16,17]. All clinical trials and retrospective studies agree that tumor control is at least equivalent to standard fractionation and the post-radiation effects are rather reduced [18-22], let alone the favorable consequences of the lower number of RT fractions (see previous paragraph). Clinical radiobiology methods and the Linear- Quadratic formulation predict a better clinical outcome with HFX. For instance, for the RT schedule of 66 Gy in 33 fractions in a T1 laryngeal carcinoma we calculate the biologically effective dose (BED) using the following formula [23]:
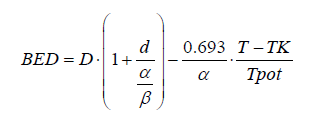
Where D: total radiation dose of 66 Gy, d: dose per fraction 2 Gy, α/β ratio= 6 Gy (the median between 4 and 8 as per Sharon Qi et al. [22]), T= 46 days (6.5 weeks), Tpot: potential doubling time= 3 days, α = 0.3 Gy-1, Tk the time by which tumor repopulation starts= 21 days [24].
If we fill in the above equation of Biologically Effective Dose the corresponding numerical values, it gives a BED=69.2 Gy6. Furthermore, if we apply the same formula for a HFX RT schedule, say that of 55 Gy in 20 fractions, (4 weeks) [16], the BED is calculated at 74.8 Gy6 or 8 % more than the BED corresponding to standard fractionation. This might correspond to at least 3% increase in TCP for a T1 or T2 vocal cord carcinoma [25]. This benefit from HFX results mainly from the reduction in treatment time from 6.5-7 weeks to 4 weeks. However, if α/β ratio was even lower, which is not unlikely, there would be a higher increase of HFX-BED probably corresponding to a higher TCP. If one applies the same LQ formula for the late reacting normal tissues, where α/β ratio equals to 3 Gy, the corresponding BEDs are 110 Gy3 and 105 Gy3 for standard and HFX schedules respectively, indicating a lower rate of late complication for the hypofrationated RT. We note here that α/β ratio, which is a measure of the fractionation sensitivity of the cells and the most known LQ parameter, traditionally has been taken as equal to 10 Gy for tumor cells [26]. However, it seems that in some tumors such as breast and prostate it can be lower [27]. For head and neck carcinomas the α/β ratio was calculated at about 8 ± 4 Gy a value favoring the use of larger doses per fraction [28,29]. Despite the above-mentioned limitations of our retrospective study, this is suggestive that there is still some room for improvement especially for T2 disease. HFX RT seems to have equivalent or even better outcomes to conventionally fractionated RT and we’re planning to employ this type of RT to our clinical practice.
All authors provided input into and approved the final draft of the manuscript.
The authors have stated explicitly that there are no conflicts of interest in connection with this article.


