Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Yan Huang, Haichen Niu and Deqin Geng*
Received: February 23, 2023; Published: March 14, 2023
*Corresponding author: Deqin Geng, Department of Neurology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, 221006, PR China
DOI: 10.26717/BJSTR.2023.49.007771
Background: Parkinson disease (PD) is a neurodegenerative disorder characterized by the massive loss of midbrain dopaminergic neurons with the motor impairments. However, a popular of hyposmia and olfactory dysfunction are found before the onset of motor impairments. It’s known that the olfactory system is the only organ that can directly contact with the environment, which is easily affected by environmental toxin than other organs. However, it’s unclear that damaging olfaction can induce the pathological response of the dopaminergic cells in the nigrostriatal system resulting in the impairment of motor.
Methods: In the present study, the neurotoxin 6-hydroxydopamine (6-OHDA) was injected into the olfactory bulb (OB) to mimic the impairing olfactory system by the environmental toxin.
Results: The results showed that the number of the tyrosine hydroxylase positive (TH+) cells in the OB was decreased 4 weeks after the 6-OHDA treatment. Importantly, it was found that the number of the TH+ cells in the substantia nigra (SN) field was also decreased. In the behavioral examination, the olfactory dysfunction and spontaneous motor impairment could be detected followed 4weeks by the 6-OHDA treatment. To study the anatomical relation between the OB and SN, the retrograde neuronal tracer, cholera toxin B (AF488-CTB), was injected in the SN to detect if the neural projection in the OB field could reach the SN in rats. It’s obvious that the CTB signal can be found in the OB field. The physical barrier (PB) was inserted into the olfactory bundle to block the OB-SN pathway.
Conclusion: Blockage of the OB-SN pathway inhibited the decreased number of TH+ cells in the SN followed by the 6-OHDA treatment. So the anatomical and physiological studies stated that the impairing OB in the physiological condition could induce the dopaminergic cell death in the SN field by the project from the OB to the SN in rats. The studies further richened the evidence that the invading olfaction by the environmental toxins will be viewed as an essential factor in the pathology of PD.
Keywords: Parkinson’s Disease; Olfaction; Substantia Nigra; Neuronal Tracing; Neurogenesis
Abbreviations: PD: Parkinson Disease; 6-OHDA: 6-Hydroxydopamine; OD: Olfactory Bulb; TH: Tyrosine Hydroxylase; SN: Substantia Nigra; PB: Physical Barrier; PFA: Paraformaldehyde; MFB: Medial Forebrain Bundle; GL: Glomerular Layer; ROS: Reactive Oxygen Species; SVZ: Subventricular Zone; DA: Dopamine
Parkinson disease (PD) is a neurodegenerative disorder characterized by the motor symptoms (bradykinesia, resting tremor, and rigidity) and non-motor symptoms [1,2]. Pathological studies found the massive loss of midbrain dopaminergic neurons and subsequent dopamine depletion in the striatum, whose changes led to the progressive, irreversible and ultimately disabling motor deficits [3]. So far it is unclear about the PD mechanism, especially, his initiation. Over the last 15 years, the numerous studies have confirmed that genetic factors contributed to the complex pathogenesis of PD. Some studies found that some vital point of some genes could induce the familial PD, which included the SNCA, Parkin, DJ-1, PINK 1, LRRK2, VPS35, LRRK2, and GBA [3-7]. But some other studies showed that the environmental exposure history with the paraquat, rotenone, manganese, and organochlorines was linked with PD [8]. The clinic observation indicated the non-motor symptoms (olfactory dysfunction, anxiety, and constipation) were found prior to the motor symptom in the PD [9]. Especially the olfactory dysfunction was a primary prodromal symptom prior to the PD diagnosis, which can be found in more than 70-80% before PD diagnosis and the onset of the classical motor syndrome [10]. Simultaneously, Braak hypothesis of PD also suggested that the alpha-synuclein pathology was firstly found in the olfactory bulb typically being associated with deficits in olfactory perception in the pre-motor phase of the disease.
Alpha-synuclein could transfer between cells resulting in distant neuropathy [11]. Based on the anatomical studies, the olfactory sensory neurons directly contacts with the environmental toxins, which can be detected by the olfactory sensory neurons and then transfer to the olfaction-relation cortex to establish the sense of smell. So the olfactory system is more liable to environmental toxins [12]. Olfactory physiological studies suggested that the olfactory bulb of mammals contained a population of approximately 5-10 % of dopaminergic interneurons within the glomerular layer, showing a distinct laminar distribution [13], in which the 10%–16% of these periglomerular (PG) interneurons were DAergic cells. The neuronal cells could be served as the initial processing center of sensory information from the olfactory sensory neural cells, namely olfactory sensory formation-related neurons [14]. Studies indicated that the endogenous DA release increased the olfactory bursting frequency regulating the olfactory behaviors. Impairing DAergic cells in the OB induced the olfactory dysfunction [15]. Previous reports about the anatomical study indicated there was an existence of a dopaminergic nigral-olfactory projection by anterograde tracing with an injection of DiI into the SNc [16]. But it’s unknown if the DAergic cells damages in the OB can impair the substantia nigra by the OB-SN projection. But so far, it’s assumed that the dopaminergic neurons in the OB are fragile and susceptive cells, whose damage will induce the dopaminergic cells death of SN filed. Thus, the present study was included: 1. Impairing dopaminergic neurons in the olfactory bulb effects on the olfactory ability. 2. Impairing dopaminergic neurons in the olfactory bulb effects on the dopaminergic cells in substantia nigra. 3. Nigral-olfactory projection from the OB to the SN.Animals:
Adult Male SD Rats (8–10 weeks) Weighing 180–220g were used in this Experiments
The number of animals in every group was 8-10. The animals were feed in the standard laboratory with a constant temperature (23 ± 2 °C) and humidity (55–60%) and 12-h dark/light cycle. Animals were permitted to freedom access the foods and water. All operation was performed in accordance with Xuzhou Medical University animal welfare and operation guiding principle.
Dopamine Depletion in Olfactory Bulb
To explore that the dopamine depletion in the olfactory bulb effects on the substantial nigral-striatum pathway, the 6-hydroxydopamine (6-OHDA, CAS Number: 7681-57-4, Sigma-Aldrich, China, Shanghai) was microinjected in the olfactory bulb of rats with the stereotaxic technique. For the stereotactic, rats were deeply anesthetized with 100 mg/kg ketamine and 15 mg/kg xylazine (i.p.) and fixed into a stereotactic frame (Kopf Instruments, CA, USA). Firstly a small skin incision was made, and a hole was drilled in the olfactory bulb on the skull (AP: +7.0 mm, ML: +1.0/-1.0 mm, DV: +4.0 mm). The coordinate is based on the rat’s brain atlas (George Paxinos Charles Watson, 7th Edition). The needle (27G) was placed in the position and, after 2 min, 2 μl of 5 mg/ml 6-OHDA in 0.09% saline or vehicle (0.09% saline) was laterally injected into the OB using a Hamilton 5 ul syringe. The injection was finished in 10 min and the needle lasting for 5 min in order to allow the solution to diffuse into the surrounding tissue and prevent the leakage.
Behavioral Tests
Open- Fields Test: The locomotor ability of different groups were detected (n=8) in the open fields’ apparatus 4 weeks after the 6-OHDA injection in OB. The open-field apparatus consisted of a square area (50×50 cm) with dark walls that were 45 cm high. For the open-fields test, animals were habituated in the open field apparatus lasting 30 min every day for 3 days. On a fourth day, animals were placed in the center of apparatus. The distance traversing in the central area and the duration of movement in the center was then traced by a video camera system for 15 min. The apparatus was cleaned in the interval of every animal.
Odor Habituation/Dishabituation Test: Odor habituation/ dishabituation of different groups was also detected (n=8) in the open fields apparatus 24hours after the locomotor test. Odor habituation/ dishabituation test was performed as the previous report [17]. A 50 cm × 50 cm × 45 cm plastic cage, with a removable plexiglass cover, was used as the testing apparatus with two glass plates placed 25 cm apart in the diagonal position. A 1.25 cm-diameter hole was drilled at the center of the cover for application of odorants. Odor (10–6v/ v, lemon or mint essence dissolved in mineral oil) was introduced on one side of the compartment, and a control odor (mineral oil) was introduced on the other side. Before the experiment began, the subjects were habituated to the box and the control odor for five minutes a day for three consecutive days (between 09:00 and 15:00 each day). On the fourth day, the habituation/dishabituation discrimination behaviors were recorded. The odor and control solutions (25 μl each) were applied to filter paper and placed on the glass plate. During the trials, the animal was first presented with the lemon odor on one side of the compartment and the control odor on the other side in five consecutive 5-min trials; each trial was separated by a 15 min break interval after acclimation. In the sixth trial, the lemon was replaced by a novel odor (mint), and the animals were subsequently exposed to the mint odor for 5 min. The amount of time that the animal spent investigating (sniffing) the mint or mineral oil was recorded. Sniffing was defined as when the animal placed its nose within 1 cm of the glass plate surface. During each experimental break interval, the compartment was cleaned with 75% ethanol. Odor discrimination was considered to be impaired if the animal spent less time investigating the new (mint) odor in the sixth trial.
Project Tracing
To label the axonal projections arising from the SNc and the OB, we stereotactically injected 200μL of the antitrade tracer was injected in the SN(AP: -5.2 mm, ML: +2.2/-2.2 mm, DV: +8.0 mm, N=8). In the experiment, the Cholera Toxin B subunit (CTB, ID: 329775212, Sigma-Aldrich, China, Shanghai) was used as the tracer. One 4 weeks later, all animals were slaughtered by transcardial perfusion with icecold phosphate-buffered saline (0.1M PBS) followed by 4 % (wt/vol) paraformaldehyde (PFA) in 0.1 mol/L PBS as previously described [17]. And then the positive signal cells were screened in the OB field to confirm the connectivity between the OB and the SNc.
Physical Blocking of the OB-SN Pathway Effects on the Dopaminergic Cells in the SN
Based on the anatomical studies, the information from the OB was transferred by the olfactory tract. To assess if the pathological factors were moved to SN by the project from the OB to SN, the olfactory trace was cut off by the physical method. In the experiment, the olfactory trace was blocked by the physical barrier as previous descriptions [16]. A sterile polypropylene sheet (2 × 6 mm) was implanted as a physical barrier into the olfactory tract to prevent the migration of pathological factors from the OB to the SN in the 6-OHDA rats. The number of TH+ cells in the SN field was further qualified to assess if the project from the OB to the SN was necessary for the migration of the pathological factors.
Statistics
Data are shown as mean ± SEM. The data were analyzed for variables of interest using the statistical package for the social sciences (SPSS v. 13.0). A paired-samples Student’s t-test was used for odor and locomotor evaluations. The immunohistochemistries data were analyzed using one-way ANOVA/independent student’s test to identify group differences. The alpha level for significance was set at 0.05.
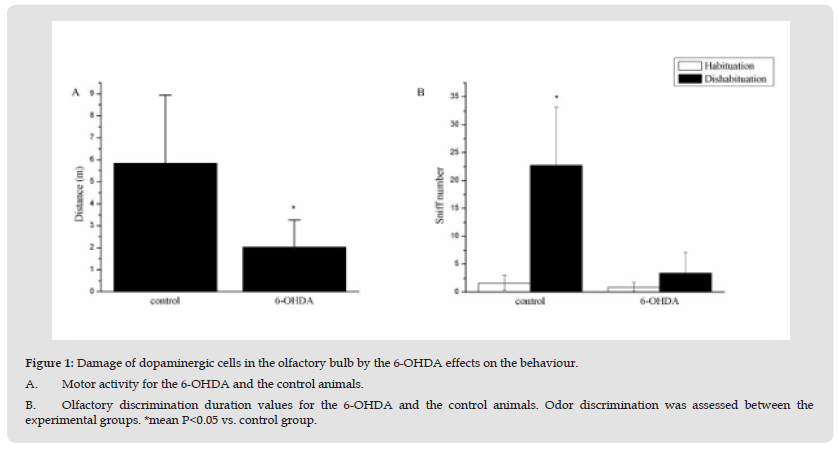
Figure 1 Damage of dopaminergic cells in the olfactory bulb by the 6-OHDA effects on the behaviour. A. Motor activity for the 6-OHDA and the control animals. B. Olfactory discrimination duration values for the 6-OHDA and the control animals. Odor discrimination was assessed between the experimental groups. *mean P<0.05 vs. control group.
Damage Of Dopaminergic Cells in the Olfactory Bulb by 6-Ohda Effects on the Behavior
In the present study, we will explore if the damage of dopaminergic cells in the olfactory bulb by 6-OHDA effects on the dopaminergic cells in the SN. Our results showed that damaging the dopaminergic neurons in the OB could impair the motor behaviors in the open field (vs. control, P<0.05) (Figure 1A & Figure 2B). The rats of 6-OHDA group (6-OHDA, 2.64±0.44m) showed the less locomotor than control rats (5.83±1.67m, vs. 6-OHDA group, p<0.05). Furthermore, the rats of OB lesion group ((2.03±0.47m) also showed the less locomotor than control animals (5.72±0.65m, vs. OB group, p<0.05). During the olfactory habituation, the 6-OHDA animals and the control animals had similar odor sniff number after 5 min delay (Figure 2A; Groups, F (1,9) = 0.1, n.s.), however during the novel odor discrimination capacity testing, the 6-OHDA animals showed the less sniff behavior than the control animals (Figure 2A & Figure 2B); Group, F (1,9) = 0.5, n.s.). The results indicated that the olfactory ability of the 6-OHDA animals was impaired by the OB treatment.
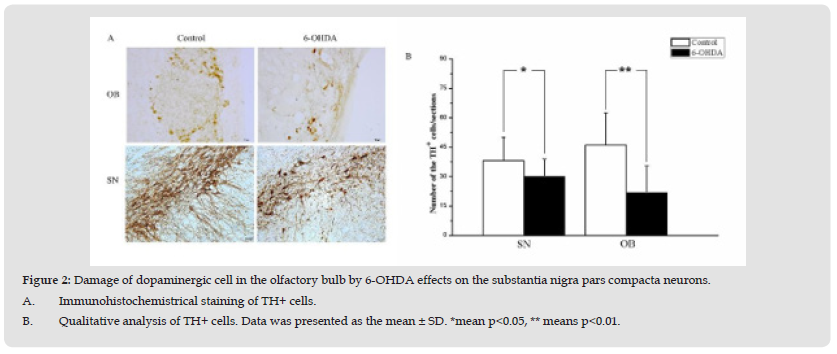
Figure 2 Damage of dopaminergic cell in the olfactory bulb by 6-OHDA effects on the substantia nigra pars compacta neurons. A. Immunohistochemistrical staining of TH+ cells. B. Qualitative analysis of TH+ cells. Data was presented as the mean ± SD. *mean p<0.05, ** means p<0.01.
Damage of Dopaminergic Cell in the Olfactory Bulb By 6-Ohda Effects on the Substantia Nigra Pars Compacta Neurons
Previous studies indicated that the 6-OHDA could be used to mainly damage the dopaminergic cells [18,19]. In order to explore if the damage the dopaminergic cells in the OB effects on the substantia nigra pars compacta neurons, the 6-OHDA is used to impair the dopaminergic cells in the OB. The results indicated that the number of TH+ cells in the OB was decreased 4 weeks followed by the 6-OHDA treatment (p<0.05). And a further analysis showed that the number of TH+ cells in the SN was also decreased followed by 6-OHDA treatment in the OB (Figure 2B) (p<0.05). On contrast, the damage of the SN by the 6-OHDA cannot induce the number change of the dopaminergic cells in the OB field (p>0.05).
Neural Project Between the Olfactory Bulb and the Substantia Nigra
To answer the anatomical relationship about the OB and the SN, we must confirm the project for them. CTB was used to trace the project between the OB and the SN. Previous studies indicated that the dopaminergic neurons located in the SNc project via the medial forebrain bundle (MFB) to the striatum, nucleus accumbens and olfactory tubercle [16]. So many dopaminergic neurons were found in the glomerular layer (GL) of the olfactory bulb [20]. In our experiment (Figure 3A & Figure 3B), the transsynaptic tracer, CTB- 488, was injected in the SN and the positive signal was found in the OB filed, showing that some individual nigral dopaminergic neurons can establish the synaptic connectivity with the neural cells from the olfactory bulb.
Physical Blocking of the OB-SN Project Effects on the Dopaminergic Cells in the SN
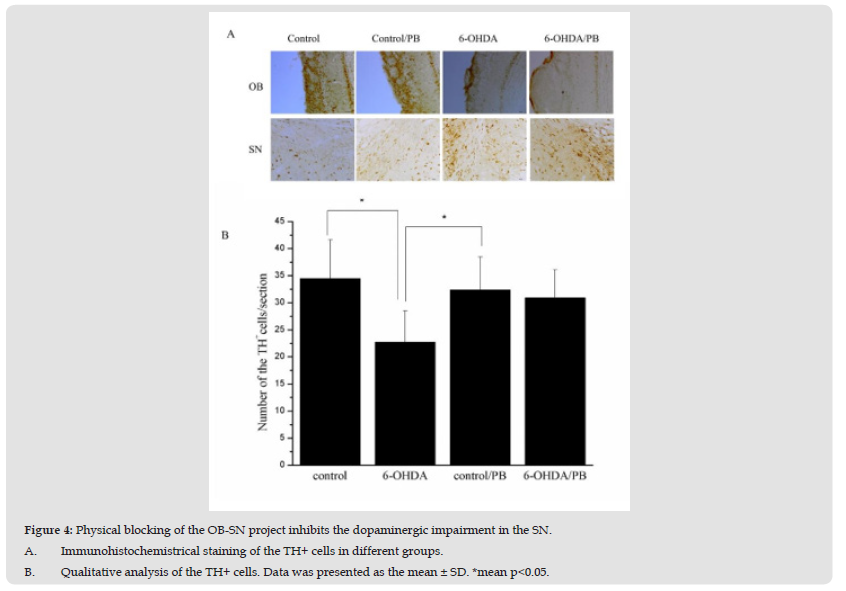
To confirm that the pathogenic factors induced by the impairing the dopaminergic cells in the OB can be transferred by the OB-SN project, the physical barrier was used to block the project. As studies showed (Figure 4A & Figure 4B), the 6-OHDA injected in the OB could induce the decreased number of the TH+ cells in the SN (p<0.05). However, the physical barrier inhibited the decrease of the TH+ cells in the SN (p<0.05). Correct anatomical location of the PB was ascertained histologically in each individual rat. The results indicated that the physical blockage of the OB-SN project could disturb the migration of the pathogenic factors in the OB.
Figure 3 Neuroal projection from the substantia nigra into the olfactory bulb by the CTB-488 tracing. A. Schematic diagram about the injection site and signal. B. Microscopic image showing that the tracer substance CTB-488 (green)was injected into the substantia nigra (SN, n=8). DAPI (blue) was used to stain the nuclei to outline the anatomical structure. Results indicated that the tracer substance CTB-488(green) injected into the SN could be transferred to the glomerular layer of the olfactory bulb (OB). arrows mean the signal of CTB-488.
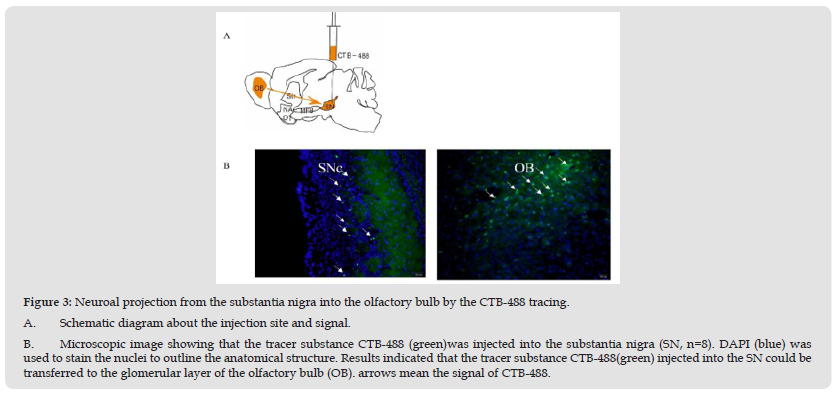
Figure 4 Physical blocking of the OB-SN project inhibits the dopaminergic impairment in the SN. A. Immunohistochemistrical staining of the TH+ cells in different groups. B. Qualitative analysis of the TH+ cells. Data was presented as the mean ± SD. *mean p<0.05.
This study finds that the 6-OHDA microinjection in the OB can impair the and also impair the dopaminergic cells of SN, which lead to the olfactory dysfunction and the impairment of motor ability, whose contributions can be blocked by the physical barrier in the olfactory tract. To further study the anatomical relation between the OB and the SN, the CTB tracing results demonstrated that the CTB signal could be found in the OB after the CTB was injected in the SN. The results indicate that the dopaminergic interneuron in the olfactory bulb is a target cell, which will be destroyed by the pathological factors leading to the dopaminergic cells death in the SN by the OB-SN project. Previous studies reported that the environmental factors, especially chemicals, are suspected to be risk factors for neurodegenerative diseases [21]. Roughly 10% of total PD cases are thought from the inherited genetic factors (single-gene mutant). However, the majority of PD cases arise from unknown causes with a long history of links to tonic environmental exposures. For the human, it’s danger exposure to the intake of such chemicals by ingestion, cutaneous contact, or inhalation. There was an association between Parkinson’s disease (PD) and the use of a group of pesticides [22]. The human could absorb the chemicals at the olfactory mucosa reach the brain directly through passive diffusion from the extra-neural space of olfactory nerves to the cerebrospinal fluid and/or by the active axonal transport of olfactory neurons [23]. But the relation between the olfactory tonic exposure and the dopaminergic cells death has not been established yet.
The olfactory bulb received the input information from the olfactory sensory cells in the olfactory epithelium and then projected the integrated information to the brain [24]. In the animal experiment, the chronic subcutaneous infusion or direct brain infusion of rotenone-induced the DA neurodegeneration in the substantia nigra (SN) and features of PD in rats, suggesting that exposure to neurotoxins such as rotenone is a risk factor for PD [25]. In our experiment, the dopaminergic cells in the olfactory bulb were thought as the susceptive candidate cells which would transfer the pathological factors to the SN by the OB-SN project. 6-OHDA was used to mimic the pathological factors that could impair the dopaminergic interneuron cells in the OB. The results were confirmed by the reduced number of the TH+ cells in the OB. The dopamine neurotransmitter was an unstable molecule that undergoes auto-oxidation to form dopamine quinones and free radicals, which was catalyzed by metals, oxygen or enzymes such as tyrosine [26]. DA neurons were vulnerable to reactive oxygen species (ROS) generated by aberrant mitochondrial respiration. The dopaminergic cells in the olfactory bulb would maybe be vulnerable target cells and easily be destroyed by the especially toxic compound. By the way, the impairment of dopaminergic interneuron in the OB also makes the animal show the olfactory dysfunction in the behavioral level. The results are consistent with the clinical observation in PD case [27]. The olfactory system is an intact organ including the olfactory epithelium, olfactory bulb, olfactory bundle and other olfactory cortex. Odor can stimulate the olfactory sensory neuron located in the epithelium, which relays the neural stimulus into the OB. After the integrating in the OB, the olfactory information is relayed to the olfaction-related cortex [28].
Impairing the epithelium can disturb the olfactory behaviors in rodents [29,30]. In the OB, the dopaminergic interneuron forms an extensive network of lateral connections that mediate cross-talk among glomeruli by releasing the DA onto sensory nerve terminals and postsynaptic neurons [31]. So the 6-OHDA could decrease the number of neurons expressing the enzyme tyrosine hydroxylase (TH) and impair the dopaminergic cells in the OB inducing the olfactory dysfunction. Subventricular zone (SVZ) neurogenesis continuously could produce the new dopamine (DA)-containing interneurons for the olfactory bulb (OB) in most adult mammals. Studies reported that the newly formed neurons were preferentially incorporated into glomerular circuits of the lesioned area to recovery the olfactory behaviors [32]. PPX is a non-ergoline D2/D3-receptor agonist that can be able to actively stimulate cell proliferation as well as adult neurogenesis in the hippocampal DG of naive mice [33]. In the anatomical studies, the previous studies reported that there was a direct axonal dopaminergic projection from the SNc to the olfactory cortex including the EPL and GCL of the olfactory bulb [16]. This result can explain that the death of dopaminergic cells in the SN results in the olfactory impairment by the project. But for our studies, the retrograde CTB signals can be found in the OB after the CTB was injected in the SN, indicating that the olfactory bulb can project to the SN field. The periglomerular dopaminergic interneurons in the olfactory bulb were believed to synthesize the dopamine neurotransmitter [34]. When the dopaminergic interneurons in the OB were impaired by the 6-OHDA, the pathological factors can be transferred to the SN. But the conclusion was needed to be further studied. However, to block the pathological factors from the OB to the SN, the physical barrier was used to block the transferring, whose results indicated that blockage of pathological factors from the OB to the SN could inhibit the dopaminergic impairment in the SN. In the present study, we provided evidence that the dopaminergic cells in the OB is impaired by the pathological factors, which can induce the NS impairment of the dopaminergic cells by the pathway from the OB to the SN.


