Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Mit Chauhan, Asma Jamil*, Richard Miller, Nayaab Bakshi and Claudia Komer
Received: February 17, 2023; Published: March 07, 2023
*Corresponding author: Asma Jamil, Saint Michaels Medical Center, Newark, NJ.111 Central Ave, Newark, NJ 07102, New Jersey, USA
DOI: 10.26717/BJSTR.2023.49.007754
Ventricular rupture and sternal wound dehiscence are two serious complications in a patient who has undergone a coronary artery bypass (CABG). Currently, only one publication has outlined ventricular perforation caused by a sternotomy wire. The following report presents and discusses a patient who underwent a CABG and post-operatively, developed sternal wound dehiscence due to excessive coughing. Following the sternal wound dehiscence, the patient suddenly decompensated during a routine position change and was found to have ventricular perforation from a displaced sternal wire. The patient survived both complications. This case reflects life-threatening complications arising in both the post-extubation phase and routine positioning change in patients undergoing CABG.
Keywords: Coronary Artery Bypass Graft (CABG); Postoperative Day (POD); Methicillin-Resistant Staphylococcus Aureus (MRSA); Cardiopulmonary Resuscitation (CPR); Packed Red Blood Cells (PRBC); Saphenous Venous Graft (SVG); Internal Mammary Artery (IMA)
Ventricular perforation is a rare but rightfully dreaded complication occurring in patients who undergo a CABG. Myocardial rupture constitutes 0.3 to 1.1% of the 900,000 emergency room cardiac trauma visits in the USA each year. The incidence of myocardial rupture on autopsy, however, is 36-65%, indicating that myocardial injury has a high probability of immediate death [1]. Ventricular perforation is most commonly caused by trauma and crush injury, followed by pacemaker placement, sternotomy wire displacement, closed cardiac massage, CPR, and sternal wire injury [2,3]. On the other hand, the incidence of sternal wound dehiscence in post-CABG patients is 0.2 to 5%, with non-infectious sternal dehiscence reported at an incidence of 0.4 to 1% in cardiac surgeries [4]. Patients with COPD, diabetes (both insulin and non-insulin dependent), and obesity with BMI > 30, smoking, female sex, and renal failure are at an increased risk of sternal wound dehiscence [5,6]. Older age, New York Heart Failure classes 3 and 4, and previous CABG have been known to increase the risk for sternal wound infections and hence, have indirectly become risk factors for wound dehiscence [7]. Additionally, the use of ACE inhibitors causing sternal dehiscence has been reported.
A seventy-eight-year-old male with a past medical history of hypertension, prediabetes, coronary artery disease, and severe aortic stenosis presented to the emergency department complaining of chest pain, which started the day before the patient’s visit. The pain was exertional, rated seven out of ten in severity, relieved by resting on the sofa, and exacerbated by movement. The patient did not use any medications in an attempt to relieve the pain. Chest pain was associated with dizziness and palpitations. Additionally, the patient also complained of chronic dyspnea. The review of systems was negative for any other complaints. The patient smoked three cigarettes per day for more than twenty years and denied drug use. He did not have any family history of heart disease, atrial fibrillation, or stroke. No allergies to any drugs were reported. The patient had significant cardiac history. His first cardiac catheterization showed significant stenosis of the right coronary artery (RCA) with stent placement. The second cardiac catheterization one month before the current visit showed 20% stenosis of the left main coronary artery, 30% to 40% stenosis diffusely in the left anterior descending artery (LAD) with 50% in the mid-section, 70% stenosis in the ostial diagonal branch 1 of LAD, 100% occlusion of left circumflex artery, 100% occlusion of the obtuse marginal artery, 70% restenosis of RCA and 50% left ventricular ejection fraction. He reported having severe aortic stenosis on a previous echocardiogram, with unknown severity as details were not available in the medical record. At that time, aortic valve replacement and CABG were recommended for symptom relief.
Upon admission, the patient was hemodynamically stable with a blood pressure of 125/60, heart rate of 64, respiration rate of 17, afebrile, and saturation of 100% on 2 liters nasal cannula. Physical exam was significant for ejection systolic murmur most prominent in the right midsternal region, S1 and S2 were present without any gallops, rubs, or thrill. The rest of the exam was normal. All labs including complete metabolic panel, complete blood count, prothrombin time, INR, urinalysis, brain natriuretic peptide, and troponin were normal. The chest x-ray did not show any pleural effusion, consolidation, atelectasis, or trauma. EKG showed inferior wall Q wave, and nonspecific T wave changes with the right bundle branch block. CABG was performed the next day, with reverse SVG sequential to diagonal, OM, distal RCA, and aortic valve replacement with # 21 mm Mosaic ultra porcine tissue valve. On postoperative day (POD) 1, 12 hrs after surgery, the patient had labile blood pressure with desaturation requiring bag-mask ventilation and a decrease in sedation. A bedside transesophageal echocardiogram was performed, which showed right-sided collection compressing right ventricle, right ventricular hypokinesis, and concern for right ventricular diastolic collapse raising suspicion for pericardial tamponade. Therefore, the patient was taken for an emergency exploratory thoracotomy. There was no intra-pericardial collection, but both lungs were swollen and touching midline. Right and left intrapleural chest tubes were placed. The sternum was not closed to prevent tamponade secondary to lung edema. Laparotomy sponges with antibiotics and vacuum dressing were applied. Slowly, the patient showed clinical improvement. Due to temperature spikes, he was treated with intravenous vancomycin, and antibiotics. Platelets and packed red blood cell transfusions were given on an as-needed basis. On POD 5, the patient underwent sternum closure, with multiple stainless-steel wires after chest and tube irrigation. A transesophageal echo was performed, which showed an ejection fraction of 40%, severe inferolateral hypokinesis, and the absence of aortic insufficiency or paravalvular leak.
Figure 1 Dancing sternal wires: lower sternal wires are displaced in our patients a few days before ventricular perforation.
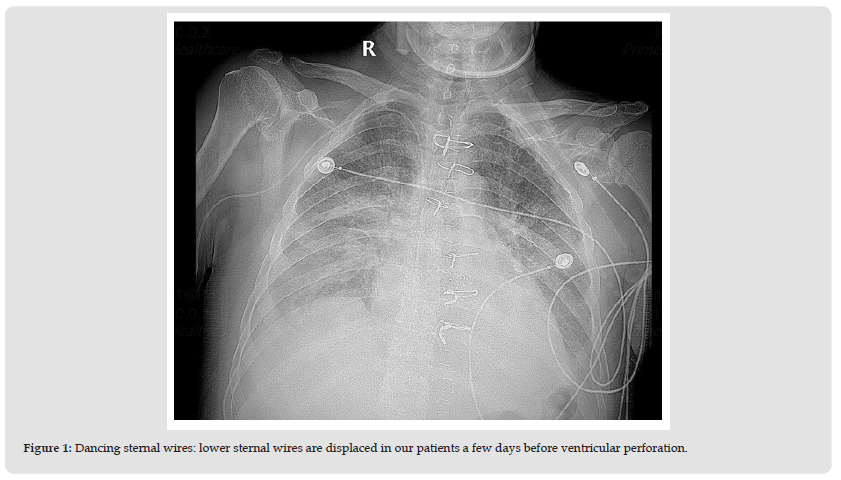
Patient sputum grew Klebsiella, after which, meropenem and vancomycin were added for hospital-acquired and ventilatorassociated pneumonia coverage. Later, vancomycin was stopped as the MRSA culture was negative and levofloxacin was added. The patient was weaned off vasopressors. The right chest tube and IABP were removed on POD 8. The patient was extubated on POD 9 to nasal cannula of 6 liters. The patient continued to have 1 mediastinal tube, 2 jackson pratt drains, and a transvenous pacemaker. He was on levophed, vasopressin, and milrinone during all this time. Post extubation, he developed a severe cough and wheezing requiring a pulmonology consult. He was started on antibiotics again along with steroids and diuresis. After multiple days, the patient’s kidney function started to deteriorate due to aggressive diuresis. On POD 18, the chest x-ray showed lower sternal wound dehiscence (Figure 1). CT chest was ordered, which showed sternal disunion likely requiring repair. The patient was intubated. On POD 19, he underwent elective re-exploration of the chest wound and insertion of bilateral chest tubes with wound-vac application to the sternum. Lower sternal wires were found to be broken and were removed. Because the patient was considered a candidate for a sternal flap, some upper sternal wires were left in place as per the request of the plastic surgeon. After the placement of bilateral chest tubes, a significant reduction in bilateral pleural effusion was noticed. POD 21, while the patient was intubated, he was turned as a part of routine cleaning.
Figure 2 CXR immediately after ventricular perforation repair by bedside. All sternal wires are now removed.
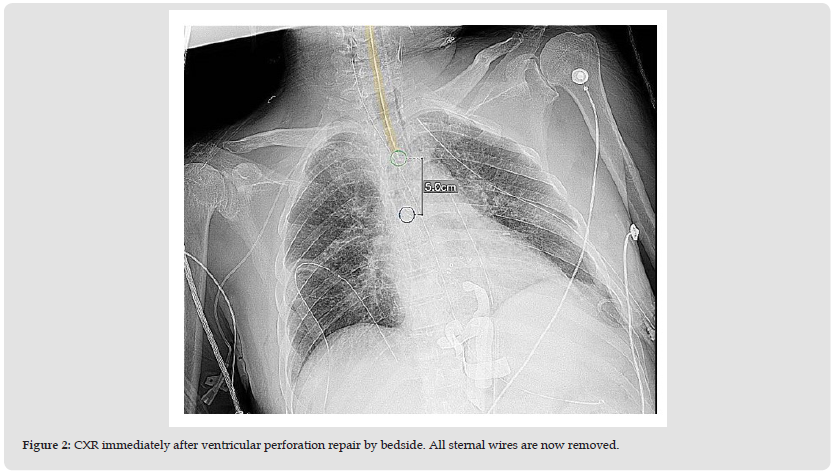
Though precautions were taken to avoid turning the upper body, the upper body was unfortunately turned as well. When the patient was laid back supine, his blood pressure suddenly dropped to systolic 40 mmHg with a massive gush of bleeding from the sternal wound and wound vac. The critical care anesthesiologist and cardiothoracic surgeon were immediately notified. An emergent left femoral sheath was placed, 1.5 liters of fluid and 3 packed red blood cells were transfused. The patient underwent bedside chest exploration by the cardiothoracic surgeon and a displaced sternal wire was found to be in the anterior right ventricular wall. The sternal wire was removed and the ventricle was repaired with 240 pledgeted sutures reinforced with bioglue. All wires were emergently removed (Figure 2). The complete mediastinum was washed with antibiotic solution, antibiotics-soaked solution pads were placed in the anteromedial sternum, and a mediastinal straight chest tube was inserted. The wound was covered with sterile dressing. On POD 25, the patient underwent a wash-out of the chest wound – with the removal of the retained laparotomy packs, irrigation of mediastinum, and repacking with antibiotics-soaked laparotomy pads. He was restarted on antibiotics, vancomycin and merrem, given the concern for a high risk of mediastinitis post-CABG. On POD 28, he was transferred to an outside facility. He underwent a right rectus flap placement and a bilateral pectoralis major myocutaneous flap closure of the dehisced wound. During surgery, patient’s sternum was found to be divided into multiple pieces. The patient recovered successfully and was extubated on POD 34. He suffered from post-operative anorexia and needed physical rehabilitation, but underwent a remarkable recovery. His sternotomy flap healed without any complications.
Sternal dehiscence is a separation of the sternum. It is an uncommon complication with an incidence of 0.2% to 5% among interventions with sternotomy access. Risk factors for sternal wound dehiscence can be divided into non-surgical and surgical causes. Non-surgical risk factors include increasing age, female sex, diabetes, smoking, malnutrition, sepsis, and osteoporosis. Surgical risk factors include poor technique, extended operative time, hypoperfusion, excessive hemorrhage, use of bone wax, internal mammary artery harvest graft, cardiopulmonary bypass, insufficient sternal fixation, and prolonged ventilation [8]. Presentation of sternal dehiscence includes pyrexia and respiratory distress. Loss of sternal alignment on chest x-ray is the most common diagnostic imaging finding, commonly called the “dancing sternal wires” sign [9]. One case report identifies persistent cough leading to sternal wound dehiscence in obese patients but it criticizes the type of suturing technique with higher wound dehiscence with a figure of 8 sutures rather than interrupted sutures in our case [5]. Our patient had risk factors such as increased age, malnutrition, and antibiotic treatment for his ventilator-associated pneumonia treatment and associated sepsis. Additionally, early reexploration for suspected pericardial effusion and persistent severe coughing postextubation due to severe fluid overload from diastolic dysfunction of the heart created sternal wound dehiscence. On the other hand, our patient acutely developed ventricular rupture or perforation in the setting of a dehisced wound. Ventricular rupture is a fatal post-CABG complication in itself. Etiologies of ventricular wall perforation or rupture can be divided into ischemic and non-ischemic. Ventricular rupture most commonly occurs in post-myocardial infarction. Nonischemic causes of ventricular rupture may be infectious, traumatic, and oncological tumors such as myxoma. Endocarditis may cause ventricular rupture as a result of myocardial phlegmon [10].
Traumatic causes of ventricular rupture can be divided into penetrating or non-penetrating (blunt). Trauma during transvenous procedures and pericardiocentesis may also cause ventricular rupture [10]. Blunt chest trauma from violent falls, motor vehicle accidents, or fist fights may lead to cardiac rupture [11,12]. Traumatic ventricular rupture usually occurs at the right ventricle apical region due to its anterior location in the thorax [13]. Deep sternal wounds following major cardiac surgery and vacuum-assisted closure therapy (VACT) used to treat such wounds have led to right ventricular rupture [14]. Surgical revision of infected sternotomy in post-CABG patients also increases the risk of right ventricular rupture [15]. Development of focal adhesion post-median sternotomy for mediastinitis may also lead to rupture of the right ventricular wall [8]. One study outlines that ventricular ruptures are seen in female patients greater than 70 years old. In a study of 3579 patients, approximately 65 fatal rupture events were reported [16]. All the patients were elderly, of low body mass index and stature, and more likely to be female. Age above 70 years and a history of prior myocardial infarction were the primary predictors of nonfatal cardiac rupture. Unfortunately, our patient had some of these risk factors as he was seventy-eight years old, had a low BMI, and had a history of prior stent placement. In most patients, ventricular rupture presents as hemodynamic instability, hypotension, cardiogenic shock, chest pain, dyspnea, cold extremities, and altered mental status [17]. These symptoms occur suddenly or within 48 to 72 hours depending on the size of the rupture. A chest x-ray may show cardiomegaly. Electrocardiograms may show ST elevations [18,17]; however, though they may demonstrate signs of rupture, EKG, troponins, and chest x-ray are not that useful in detecting traumatic myocardial rupture and CT chest remains the diagnostic tool of choice. Because our patient developed acute hemorrhagic shock with bleeding into the wound vac and from the sternal wound, emergent exploration was the only option. Imaging or lab studies were unable to be performed until after the intervention, which revealed the rupture itself. Our patient is unique in two ways. He suffered from two of the rarest and most devastating post-CABG complications: sternal wound dehiscence as a result of excessive coughing and right ventricular perforation from displaced sternotomy wire.
The events leading to both complications were uncommon and difficult to predict. Due to timely recognition and intervention, the patient had a successful recovery, despite prolonged hospitalization. This case also outlines the role of routine patient care nursing practices and their implications on patient safety and outcomes. Physical manipulation to clean the patient and turning of intubated patients every two hours are routine nursing practices in an acute care setting, but care should be taken in patients with sternal dehiscence with wires in place. Furthermore, the direct right ventricular injury was due to the sternal wires, rather than the sternum itself. Once ventricular injury was identified, treatment was straightforward with the repair of the rupture myocardium. Usually, the management of sternal dehiscence requires a multi-pronged approach. Procedures include, but are not limited to tightening of sternal wires, placing extra sutures on the sternum and at skin closure, and muscle flap (pectoralis, rectus, omental, latissimus dorsi flaps) to better stabilize the sternum [19]. Since our patient had sternal exploration four times, the patient’s sternum had been divided into pieces. All wires were removed after ventricular perforation and the pectoralis myocutaneous flap was performed resulting in remarkably good outcomes. There was concern about decrease mineral bone density as all wires were found to be displaced.
In patients who have undergone a CABG, extubation should be performed cautiously because early extubation can lead to both high preload and afterload and an increase in cardiac strain, resulting in respiratory failure, requiring reintubation, and causing short-term sternal wound dehiscence. In our patient, who had a dehisced sternal wound, a simple physical turning maneuver resulted in sternal wire dislocation leading to right ventricular perforation. Such cardiac injury due to physical manipulation is not frequently encountered. This case also raises the question about the type of suturing technique that should be applied in post-sternotomy wounds. There is no single suturing technique specified that is superior to others to prevent wound dehiscence. Therefore, we suggest that protocols should be initiated emphasizing special training in post-operative care in cardiac rehabilitation units concerning moving and turning patients. A high degree of suspicion should be present to detect fatal complications of ventricular perforation in a dehisced sternal wound with wires in place in patients who have undergone a CABG. Only prompt recognition and timely intervention can save a patient’s life and prevent worse outcomes.
The authors have no conflicts of interest to declare.
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
We are thankful for the support of the patient for allowing us to publish this case report. We also acknowledge the love, support, and guidance of our attendings, Dr. Richard Miller and Dr. Claudia Komer in writing this case report.


