Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Anwarul Islam*
Received: February 25, 2023; Published: March 02, 2023
*Corresponding author: Anwarul Islam, M.D., Ph.D., FRCPath., FACP, Attending Physician Division of Hematology, Oncology Department of Medicine, Buffalo General Medical Center, Buffalo, New York, USA
DOI: 10.26717/BJSTR.2023.49.007740
Bone marrow aspiration is an invaluable tool in the characterization of various clinical conditions and is extensively used in hematology and oncology. Conventionally, it involves the cytology of bone marrow cells smeared on a slide, their subsequent staining with a Romanowsky stain, and their careful examination under a bright field microscopy. We have studied freshly prepared bone marrow aspirate smears and compared them with smears made from a sample (obtained at same time and standing at room temperature) at regular intervals up to 24 hours post-aspiration. Our study demonstrates that prolonged standing causes morphologic changes that mimic specific features of myelodysplasia and may interfere with accurate interpretation and diagnosis.
Keywords: Cytology; Morphology; Cytomorphology; Bone Marrow; Morphologic Changes Due to Prolonged Standing
Examination of bone marrow aspirate smears plays an important role in the diagnosis and management of patients with various hematological disorders. Ideally, bone marrow aspirate smears should be made immediately following the withdrawal of the marrow from the patients [1,2]. However, this is not always the case, and in practice, bone marrow samples are often collected by the clinical staff (hematologist, oncologist, interventional radiologist, and nurse practitioners) and sent to the laboratory after a variable period of delay. By the time the marrow samples arrive in the laboratory (often several hours) degenerative changes in hematopoietic cells have already begun. As a result bone marrow aspirate smears prepared from such samples are prone to show morphological changes in erythroid and granuloid precursor cells as well as megakaryocytes. To ascertain the type and extent of morphological changes that occur due to prolonged standing we have studied BM aspirate specimens from patients diagnosed with various hematological disorders which have been left standing for 30 minutes to 24 hours. Smears were made at regular intervals and the stained smears were examined to see what morphologic changes had developed during the intervals. It was observed that the longer the marrow specimens were kept standing before the smears were made the more pronounced the morphologic changes. Some of the changes that were noted could mimic the alterations seen in patients with myelodysplastic syndromes [3]. We recommend that whenever it is possible the smears should be made fresh and actively air-dried immediately after the sample is withdrawn from the patient. This approach helps ensure the ideal morphology of hematopoietic cells and their accurate analysis for hematological diagnosis and may even prevent misdiagnosis for conditions such as myelodysplastic syndromes (MDS).
Bone marrow aspiration and preparation of smears. Bone marrow aspirate samples were obtained from the right or left posterior iliac crest using a bone marrow aspiration needle employing methods previously described [4]. Samples were obtained from twelve patients (nine men and three women, age range 50-70 years) presenting with various hematological disorders who underwent BM aspiration and BM trephine biopsy procedure as part of their diagnostic work-up. Clinical indications included pancytopenia (n=3), leukopenia (n=2), thrombocytopenia (n=2), leukocytosis (n=2) thrombocytosis (n=1), and lymphocytosis (n=2). A first draw of 2.0 ml of BM aspirate sample was obtained using a conventional aspiration needle from the posterior iliac crest using a 5.0 ml plastic syringe. Following the BM aspiration a drop of marrow was immediately placed on each of the three glass slides and smears were carefully made after suctioning off the extra blood with a glass pipette. The smears were immediately air-dried by waving the slides in the air. The rest of the marrow was then placed in a tube containing appropriate concentrations of dipotassium ethylenediaminotetraacetic acid (EDTA). As it is known that excessive EDTA can cause artificial morphologic changes in hematopoietic cells in the bone marrow samples [5] 2 ml of marrow was collected and placed in a ‘lavender-top’ blood collection tube containing the ideal amount of EDTA (1.8 mg/ml).
The subsequent smears (three slides from each patient) were made from the collected sample (each time after gently but thoroughly mixing the sample) at intervals of 30 minutes, one hour, two hours, four hours, eight hours, 12 hours 18 hours, and 24 hours. When all the slides were prepared they were stained with Romanowsky stain in a Siemens slide Stainer (Figure 1). They were now ready for microscopic examination.
Examination of the smears. The initial examination of the smears was carried out with an overall assessment of the marrow film with an examination under a low-power (X 10 objective). This ensured that the large cells such as megakaryocytes were not missed. This was followed by examination under greater magnification including under an oil immersion lens. Careful examination of the morphologic appearance of all hematopoietic cells was made in the trails of cells commencing from a marrow fragment in the distal end (feather edge) of the smear and working back towards the head (beginning) of the film. It is known that the morphology of hematopoietic cells that are close to the fragments from which they originate is most optimal [4]. Five hundred consecutive cells in each slide were assessed and the number of cells exhibiting dysplastic changes was recorded. The following quantitative scoring technique was employed.
• None = little or no dysplastic morphologic changes were observed in any of the cell lines studied.
• Mild = less than 10% of the cells showed dysplastic morphologic changes in any or all of the cell lines studied.
• Moderate = 10- 30% of the cells showed dysplastic morphologic changes in any or all of the cell lines studied.
• Marked = More than 30% of the cells showed dysplastic morphologic changes in any or all of the cell lines studied.
Striking morphological differences were observed between the smears made of fresh marrow as compared to marrows left standing for a “lengthy” period. No noticeable changes in the morphology of hematopoietic cells were observed in the films made from the marrow which was standing for not more than an hour at room temperature and were not easily distinguishable from the films made immediately after collection of the sample of marrow (fresh marrow). By two hours changes were discernible and by 18-24 hours they became striking, affecting the morphology of all the hematopoietic cells (myeloid precursors, mature neutrophils, monocytes, lymphocytes, erythroid precursors and megakaryocytes) (Table 1). Myeloid cells were affected uniformly; their nuclei stained more homogeneously than in the fresh marrow, the nuclear lobes became separated and the cytoplasmic margins appeared ragged or less well-defined. Small vacuoles appeared in the cytoplasm and they became more prominent and more numerous and the number of cytoplasmic granules decreased the longer the samples stood. Nuclear hypolobulation (pseudo-Pelger-Huet anomaly) and bizarre nuclear shapes were also observed (Figures 2A-2D). Erythroid precursors were also affected by prolonged standing which led to progressive degenerative changes such as nuclear lobulation and budding (Figures 3A- 3B), nuclear cytoplasmic asynchrony, karyorrhexis, cytoplasmic vacuolation, and cytoplasmic blebs. These changes became more prominent and the cytoplasmic vacuolation increased the longer the samples stood before making the smears. Cytoplasmic inclusions were not seen. Megakaryocytes were also adversely affected and showed morphological changes in the form of multi-nuclearity and less granulation (Figures 4A-4B).
Table 1: The following morphologic features were looked for in the erythroid and granuloid precursors and me gakaryocytes following prolonged standing.
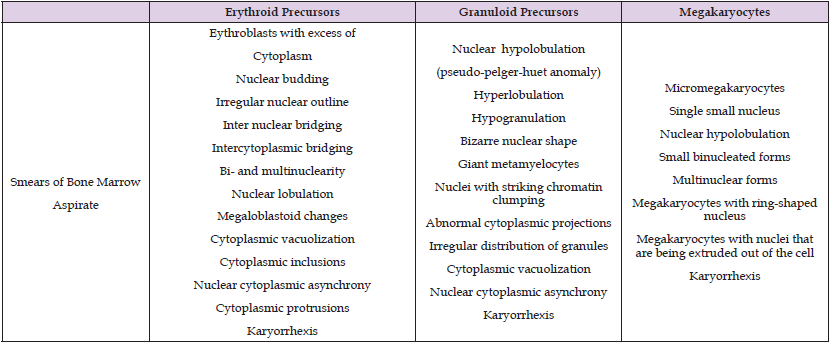
Figure 2: Morphologic changes observed in the myeloid series in the bone marrow smears prepared after prolonged standing (24 hours) in a neutropenic patient. Note the nuclear and cytoplasmic vacuolation (A), nuclear fragmentation (B), bilobed forms (pseudo Pelger-Huet anomaly) (C), and multi-segmented and bizarre nuclear forms (D).
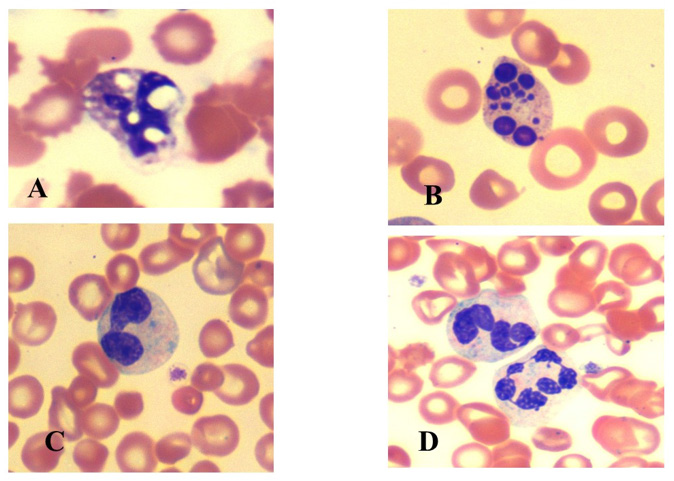
Figure 3: Morphologic changes observed in the erythroid series in the bone marrow smears prepared after prolonged standing (24 hours) in a patient with pancytopenia. Note bi-nuclear forms (A), lobulation and nuclear budding (B).
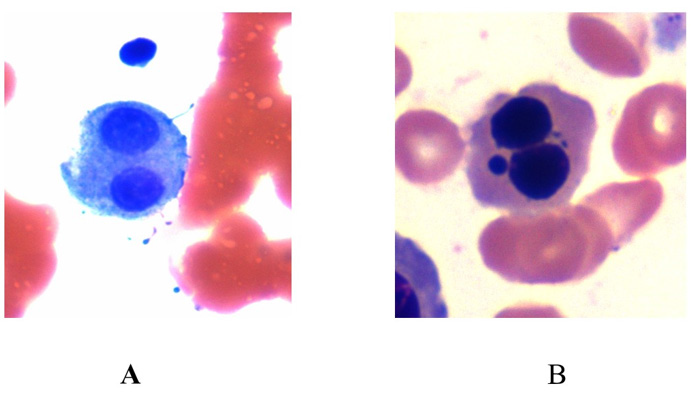
Figure 4: Morphologic changes observed in the megakaryocyte series in the bone marrow smears prepared after prolonged standing (24 hours) in a patient with thrombocytopenia. Note multi-nuclear megakaryocytes (A & B).
Examination of a BM aspirate smear is a highly informative hematological tool at the hematologist’s disposal in screening, diagnosis, treatment, monitoring of disease progression, and therapeutic response. Adequate preparation and thorough examination of a good quality smear for proper evaluation, interpretation, and diagnosis of hematological disorders cannot be over-emphasized. However, the preparation of a good quality BM aspirate smear and the aberrations in the morphology of hematopoietic cells caused by a delay in smear preparation is not universally recognized. Our findings demonstrate that the morphology of hematopoietic cells in the BM aspirate deteriorates considerably during prolonged standing. The cytological features revealed by the Romanowsky stain demonstrated that the morphology of cells was well preserved in freshly aspirated marrow but continued to deteriorate the longer the marrows were allowed to stand. All marrow (hematopoietic) cells were affected almost equally. It is recognized that degenerative changes affect the morphology of blood cells (leukocytes and red cells) when a blood sample is allowed to stand in the laboratory before blood films are made [6,7]. However, there are no reports of morphologic changes that affect the bone marrow cells when they are allowed to stand for an extended period during their transport and awaiting smear preparation in the laboratory. Sometimes if the sample arrives in the laboratory late in the day or at an inconvenient time such standing can be quite long and may even exceed 24 hours.
We have carefully studied the morphologic changes that occur between the smears made from fresh marrows as compared to smears made from marrows left standing for an extended period of time. Smears made from marrows left standing for a prolonged period (18-24 hours) exhibited marked morphological changes when examined by conventional cytological methods [8]. Some of the observed changes are described in detail in table 1 and illustrated in the photomicrographs (Figures 2-4). Most interestingly, some of the changes observed in the hematopoietic cells after prolonged standing mimicked changes that are also observed in the marrows of patients diagnosed with myelodysplastic syndrome [3], raising the question as to how often the myelodysplastic changes observed in the smears of BM aspirate specimens from these patients are true myelodysplastic changes (as part of the disease process) or that have occurred due to prolonged standing of the marrow before the BM smears were made. Although it would be unwise to draw any sweeping conclusions from a purely cytomorphological study, our results do suggest that deterioration in the morphologic appearance of hematopoietic cells does occur when the marrow is left standing for a prolonged period of time. All marrow (hematopoietic) cells were affected equally. It is recognized that degenerative changes affect the morphology of blood cells (leukocytes and red cells) when the blood is allowed to stand in the laboratory before films are made [6,7]. However, there are no reports of morphologic changes that affect the bone marrow cells when they are allowed to stand for a prolonged period of time during their transport and when they arrive in the laboratory before the BM aspirate smears are prepared. Sometimes if the sample arrives in the laboratory late such standing and preparation of smears can be quite long and may even exceed 24 hours.
It has long been known that before any stained smears can be used for hematologic diagnosis, it is important that proper technique is used in the collection, preparation, and staining of BM smears [6]. If the BM is collected properly but left standing for a prolonged period of time before the smears are made or if the smears are poorly stained, such smears may have no value, and worse still, maybe seriously misleading particularly when a disorder, such as myelodysplastic syndromes is considered [3]. To retain excellent morphology of hematopoietic cells in a bone marrow aspirate smear and avoid any ambiguity or confusion about the true morphological identity of hematopoietic cells we recommend smears should be made immediately or as soon as possible following the withdrawal of the marrow, instantly air dried and properly stained with a Romanowsky stain for hematological diagnosis. Preferably at the bedside and before the specimens are sent to pathology. In addition, hematologists and hematopathologists should be careful in diagnosing a condition as myelodysplastic syndrome or include the term myelodysplasia without knowing how long the bone marrow was kept standing before the smears were made.
I am grateful to Andrew Lange and Karen Ernst and other hematology technical staff who prepared the bone marrow aspirate slides at specific and irregular times of the day and evenings.


