Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Nina Jančar1*, Urška Kocutar2 and Martin Štimpfel1
Received:February 13, 2023; Published:March 01, 2023
*Corresponding author: Nina Jančar, Department of Human Reproduction, Division of Gynecology, University Medical Centre Ljubljana, Ljubljana, Slovenia
DOI: 10.26717/BJSTR.2023.49.007733
The main purpose of our study was to compare the parameters of women, treated with letrozole, who became pregnant after frozen embryo transfer (ET) with those who did not. We tried to determine possible prognostic factors linked with significant prospect of conception. The results of 60 women undergoing letrozole treatment performed at the Department of Human Reproduction, Division of Obstetrics and Gynecology, University Medical Centre Ljubljana were retrospectively compared with respect to the follicle size, endometrial thickness, LH peak, LH peak in relation to day 0, and FSH, estradiol, and progesterone levels in the time of LH peak. Furthermore, we have retrospectively compared the results of our study to the results of a total of 621 frozen ET gathered at our Department from beginning of January until the end of December 2018. In 269 cases natural cycle, and in 352 artificial cycle with endometrial preparation with estrogens and progesterone was used. Clinical pregnancy rate and live birth rate per ET was higher in letrozole group than in natural or artificial cycle. Miscarrige rate per ET was higher in letrozole group than in natural cycle, but lower than in artificial cycle. In conclusion, no significant difference between hormone levels, endometrial thickness, day 0 and follicle size was observed between the group of women, treated with letrozole, who did become pregnant with those who did not. No significant difference was also observed between letrozole group and natural or artificial cycle. Nevertheless, our results are promising but limited with small sample size.
Keywords: Infertility; Letrozole; Prognostic Factors; Frozen-Thawed Embryo Transfer
Abbreviations: FSH: Follicle-Stimulating Hormone; LH: Luteinizing Hormone; HCG: Human Chorionic Gonadotropin; HRT: Hormone Replacement Therapy; CC: Clomipfene Citrate; ET: Embryo Transfer
Frozen embyo transfers (FET) are widely used in assisted reproduction to treat infertility. They enable the excess embryos to be stored and utilized later. FET are used in women in whom a fresh embryo transfer does not result in a pregnancy or in those who return for another baby [1]. In contrast to the complex IVF protocols, frozen embryo transfers are relatively simple, with the main aim to sufficiently prepare the endometrium to receive the thawed transferred embryos [2]. FET also increases pregnancy rate, reduces cost and can be completed in shorter period as repeated ‘fresh’ cycles [3]. However, despite the growing importance and number of FET, the uncertainty, as to which type of cycle regime is superior, remains. After controlled ovarian hyperstimulation with gonadotrophins, many cycles result in supernumerary frozen embryos. Furthermore, with the increasing number of freeze all cycles, the optimal endometrial preparation for frozen embryo transfer (ET) has become an important matter of debate in recent years [4]. Frozen embryo can be transferred in natural cycle, in stimulated cycle, or in cycle with endometrial preparation with estrogens and progesterone. Since stimulation of ovaries for frozen embryo transfer with gonadotophins carries additional cost, stimulation with letrozole has been a good alternative in recent years [5]. Letrozole, a third-generation aromatase inhibitor, is used to stimulate ovulation in women who have ovulation disorders, such as polycystic ovarian sindrome. It usually leads to mono-ovulatory cycle and it increases the endometrial receptivity, which has a positive effect on embryo implantation [6]. In this article we have compared the parameters of the women who got pregnant after frozen ET with those who did not. We tried to assess possible prognostic factors associated with a significant likelihood of conception. In the discussion we compare the results of our study with results gathered at the Department of Human Reproduction, Division of Obstetrics and Gynecology, University Medical Centre Ljubljana in 2018. The results are also compared with other studies.
Study Design and Participants
Our study was performed at the Department of Human Reproduction, Division of Obstetrics and Gynecology, University Medical Centre Ljubljana. The study group included infertile women, who were candidates for frozen embryo transfer from April to November 2019. We included 60 consecutive women with normal menstrual cycles or polycystic ovary syndrome. Women with hypogonadotropic hypogonadism or premature ovarian failure were excluded. Letrozole was prescribed to all women in study group according to standard protocol used for stimulation of ovulation [7]. All women, who had frozen ET from beginning of January until the end of December 2018, were included in the control group. The data for these cycles was collected from the study center’s institutional database of assisted reproductive technology procedures. The collection and analysis of these data in anonymized form is allowed by the Personal Data Protection Act (Article 17, Official Gazette of the Republic of Slovenia No 94/07, 2004) and by the Healthcare Databases Act (Official Gazette of the Republic of Slovenia No 65/00, 2000; No 47/15, 2015; 31/18, 2018). The National Medical Ethics Committee of Slovenia (0120-174/2018/6) also allows the collection of anonymized data for observational study in standardized treatments in the usual management of patients. Before starting the treatment, each patient signs an informed consent for the procedures and allows data collection and analysis in anonymized form for research purposes.
Protocol
Letrozole (Femara) was given orally, 1 tablet (2.5 mg) twice a day for 5 days from the 3rd to the 7th day of the menstrual cycle. Blood samples were taken daily for Follicle-stimulating hormone (FSH), Luteinizing hormone (LH), estradiol and progesterone concentration assessment from 10th day of the cycle, until the disappearance of the follicle. We performed a vaginal ultrasound examination daily. We waited for the lead follicle to rupture on its own, but in seven subjects ovulation had to be trigged by recombinant human chorionic gonadotropin (hCG) (Ovitrelle). All patients received lutheal phase support with vaginal progesterone from day 0 with 200 mg of micronized progesteron (Utrogestan) 3 times daily. Embryo transfer was performed on day 5, after 5 days of vaginal progesterone. One or two blastocysts were transferred to uterine cavity with soft embryo transfer catheter (Cook). The positioning of the catheter tip was checked with transvaginal ultrasound immediately before the injection of embryo in small amount of growth media into the uterine cavity. Pregnancy was assessed with serum ßHCG concentration on day 15 after the transfer and clinical pregnancy was confirmed 14 to 21 days after positive pregnancy test with transvaginal ultrasound.
Parameters
Day 0 was considered the day of ovulation and in those who received recombinant hCG (Ovitrelle), day 0 was 36 hours after drug administration. Live birth was defined as the birth of a live baby on after 22 gestational weeks. Clinical pregnancy was defined as the presence of at least one gestational sac in the uterine cavity on ultrasound examination. Miscarriage rate was defined as the loss of a clinical pregnancy before the 22nd weeks of gestation. The study compared the parameters of women who became pregnant with those who did not. We evaluated the follicle size, endometrial thickness, LH peak, LH peak in relation to day 0, and FSH, estradiol and progesterone levels at the time of LH peak.
Statistical Analysis
Pearson’s chi-squared and Mann–Whitney U-tests were used to analyze the data as appropriate (the normality of data was analyzed with the Shapiro–Wilk test). Where appropriate, numerical data are presented as median and interquartile range, and P-values <0.05 were recognized as statistically significant. Statistical package SPSS was used for computer analysis.
Results
The age of the subjects ranged between 25 and 41 years with an average age of 33.78 ± 3.6 years. The average body mass index was 24.77 ± 5.54 kg / m2. A tubal factor of infertility was detected in 16 women, 17 women had endometriosis, 7 polycystic ovary syndrome, one had thyroid disease and 4 women were older than 40 years. In the subjects, the course of cycles was assessed by ultrasound. On average, day zero (0) was the 15th ± 2.12 day of the cycle (day 12- 21). The mean follicle size was 24 ± 3.20 mm (19 - 31 mm) and the endometrial thickness was 10 ± 2.38 mm (6 - 18 mm). 45 subjects or 75% received one embryo and eleven subjects or 18.3% received two embryos. There was no embryo transfer in four or 0.7% of subjects. For two of those subjects the procedure had to be canceled, due to no leading follicle, one woman started bleeding on the day of scheduled ET and for one, the single cryopreserved embryo had not survived. 21 subjects became pregnant, accounting for 37.5% of pregnancies per embryo transfer. 7 out of 21 subjects then had a miscarriage, so the proportion of miscarriage is 33.3%. 14 of the 21 subjects gave birth. The live birth rate was 25% per embryo transfer. Blood samples were taken daily for FSH, LH, estradiol, and progesterone concentration assessment. Hormonal investigations showed that the LH peak occurred on average 1.42 ± 0.88 days before day “0” (0-4 days). On average, the LH peak was 39.21 ± 22.00 E / L (16 - 98.6 E / L).
The mean FSH on the day of the LH peak was 10.51 ± 6.74 E / L (2.8 - 27.8 E / L), estradiol 1.19 ± 0.76 nmol / L (0.21 - 3.21 nmoL / L) and progesterone 4.66 ± 2.98 nmol / L (1 - 16.9 nmol / L). 7 subjects received recombinant hCG (Ovitrelle) to induce ovulation, three became pregnant but later had a miscarriage.The limitation of the study is that we did not obtain all of the data for 9 participants, as they either did not attend regular check-ups or we did not get the complete data for blood hormone levels because of the holidays or weekends.
Comparison Between Women Who Became Pregnant with those Who Did Not
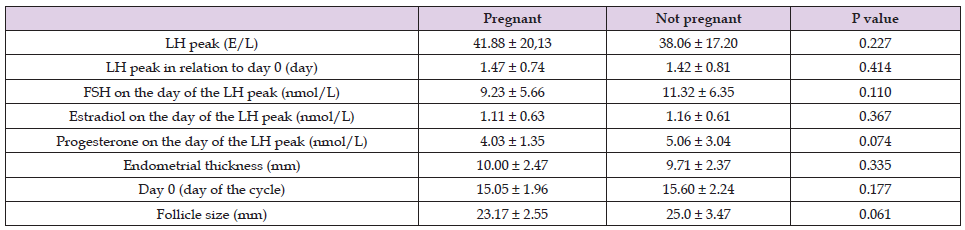
As presented in Table 1, LH peak and LH peak in relation to day 0 were similar in both groups. FSH, estradiol and progesterone concentration levels were lower in the group of women who did get pregnant, but the significant difference was not proven. Endometrial thickness was similar in both groups. The distribution of day 0 was also comparable between both groups. Follicle size was smaller in the group of women who did get pregnant but the difference did not reach statistical significance.
Table 1. Comparison between the women who did get pregnant whit those who did not after letrozole treatment.
Comparison Between the Results of Frozen ET Procedure using Natural Cycle, Artificial Cycle and Letrozol
As presented in Table 2 Clinical pregnancy rate and live birth rate per ET was higher in letrozole group than in natural or artificial cycle (37.5% versus 27.6% versus 26.6% respectively for clinical pregnancy rate and 25% versus 22.2% versus 14.7% respectively for live birth rate). Miscarrige rate per ET was higher in Letrozole group than in natural cycle, but lower than in artificial cycle (33.3% versus 20.6% versus 43.2% respectively). Miscarrige rate per ET was significantly lower and live birth rate per ET was higher in natural cycle group than in artificial cycle group. No significant difference was also observed between letrozole group and natural or artificial cycle.
Table 2. Comparison between the results of frozen ET procedures using natural cycle, artificial cycle and letrozole.
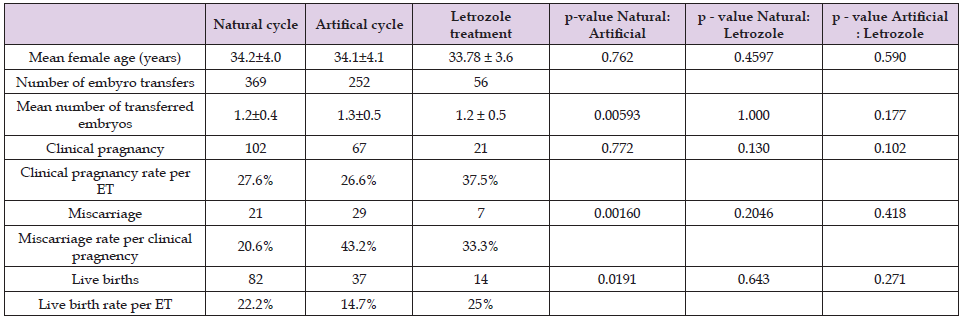
This study was carried out in order to compare the parameters of women, treated with letrozole, who got pregnant after frozen ET with those who did not. No significant difference between hormone levels, endometrial thickness, day 0 and follicle size was observed. Nevertheless, our study demonstrated high live birth and pregnancy rates along with low miscarriage rate per embryo transfer. In year 2018, a total of 621 frozen embryo transfers have been performed at the Department of Human Reproduction, Division of Obstetrics and Gynecology, University Medical Centre Ljubljana. In 269 cases natural cycle and in 352 artificial cycle was used to prepare the endometrium. As presented in table 2 clinical pregnancy rate was 27.6% in natural cycle group and 26.6% in artificial cycle group opposed to 37.5% in letrozole group in our study. Live birth rate per embryo transfer (ET) was 22.2% in natural cycle, 14.7% in artificial cycle and 25% in letrozole group. Miscarriage rate was 20.6% in natural cycle, 43.2% in artificial cycle as opposed to 33.3% in letrozole group. The similarities between the groups in terms of their demographics and histories make this comparison more valuable, as the groups were homogenous. In literature, different results comparing artificial, natural and modified natural cycles have been published. Kalem, et al. [5] who used natural and hormone replacement therapy (HRT) cycles in the preparation of the endometrium in thaw cycles, suggested similar pregnancy and live birth rate between groups.
In our study patients undergoing treatment with letrozole obtained higher clinical pregnancy rate per embryo transfer than patients undergoing HRT and natural cycles in study by Kalem, et al. [5] (37.5% vs. 19.3% vs. 23.6% respectively). Similarly, live birth rate per embryo transfer was also higher in treatment with letrozole in our study (25%) than in HRT cycle (18.8%) and in natural cycle (21.5%) in study by Kalem, et al. [5]. In a retrospective study by Jouan, et al. [7] in which 2296 patients were examined, the authors reported 24.3% pregnancy rate per transfer in Clomiphene citrate (CC) group and 14.6% in artificial cycle group (versus 37.5% our study). The ongoing pregnancy rate was 28.6% in CC group and 14.6% in artificial cycle group (versus 25% in our study) [8]. Similar findings were observed in a double blind study performed by Amer, et al. [8] where they compared letrozole treatment to CC treatment in sub fertile women with polycystic ovary syndrome. They have concluded that pregnancy rate was significantly higher in letrozole group than in CC group [9]. Study by Legro, et al. [9] suggests that women treated with letrozole also have more live births than those treated with CC [10]. However, in the study by et al, where they investigated the efficiency of letrozole treatment on ovulation induction in frozen-thawed embryo transfer, they have found no significant difference on live birth and clinical pregnancy rate per embryo transfer between the letrozole and artificial cycle group Zhang, et al. [5]. The results of our study are therefore promising but limited by small sample size.
Treatment with letrozole generally leads to mono-ovulatory cycles [11], which we have also noticed in our study. In most cases, we have witnessed spontaneous ovulation and sufficient endometrial thickness with a mean of 9.79 mm. Letrozole treatment also has other benefits. Miller, et al. [11] demonstrated that letrozole improved integrin expression which might result in higher implantation and pregnancy rates among women with endometrial receptivity defect [12]. Multiple studies suggested that letrozole use during in vitro fertilization treatments does not increase the risk of major and minor congenital anomalies [13,14]. Multiple studies also observed a decreased risk of miscarriage in the letrozole group relative to comparison groups [5,13]. Letrozole could therefore be a good lowcost infertility treatment alternative. We acknowledge that this study has limitations. We were not able to obtain all of the data for nine of 60 participants as they either did not attend regular check-ups or we were not able to perform blood hormone concentration analysis due to holidays. Furthermore, the results are limited by small sample size. In conclusion, this study compared the parameters of women, treated with letrozole, who became pregnant with those who did not. Our results showed no significant difference between hormone levels, endometrial thickness, day 0 and follicle size between the groups. Further, bigger scale studies, are therefore needed, to assess possible prognostic factors associated with a significant likelihood of conception.


