Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Panagiota Chatzivasiliou1#, Andreas Efstathiou1#, Areti Augoulea1, Anastasia Palaiologou1, Lorena Kontou1, Iliana Karagkouni1, Virginia Lampropoulou1, Achilleas Chatziioannou2, George Kaparos3, Andreas Alexandrou1, Eleni Armeni1,4$ and Irene Lambrinoudaki1$*
Received: February 13, 2023; Published: February 27, 2023
*Corresponding author: Irene Lambrinoudaki, Menopause Clinic, Second Department of Obstetrics and Gynecology, Aretaieio Hospital, University of Athens, Greece
DOI: 10.26717/BJSTR.2023.48.007729
Introduction: Non-alcoholic fatty liver disease (NAFLD) indexes, like Fibrosis-4 (Fib-4) score and NAFLD fibrosis score (NFS) can be used for the exclusion of advanced fibrosis. On the other hand, the menopausal transition has been associated with an accrual of cardiometabolic risk factors, which contribute to the risk for NAFLD and low handgrip strength (HGS). We aimed to assess the possible association between Fib-4 score and NFS and HGS, in a sample of postmenopausal women.
Methods: This cross-sectional study evaluated 122 postmenopausal women. Fasting blood samples were obtained for biochemical and hormonal assessment. The Fib-4 score and NFS values were calculated. We measured HGS values, and defined dynapenia as HGS < 16 kg.
Results: Univariate analysis showed that Fib-4 score values correlated with HGS (r-coefficient= -0.213, p-value=0.034) and dynapenia (r-coefficient=0.232, p-value=0.020). NFS also correlated with HGS (r-coefficient= -0.247, p-value=0.015) and dynapenia (r-coefficient=0.219, p-value=0.032). Both scores were correlated with age, menopausal age, body mass index and blood lipids. Women with dynapenia vs those with normal muscle strength had higher values of Fib-4 1.34±0.6 vs 1.1±0.37, p-value=0.016 ANOVA). Multivariable regression analysis showed that Fib-4 measures were linearly associated with dynapenia (b-coefficient=0.230, p-value=0.022). Logistic regression models showed that dynapenia was associated with an increment in Fib-4 values (odds ratio=5.580, p-value=0.010). All models were adjusted for age and cardiovascular risk factors.
Conclusion: Fib-4 score measures are more consistently associated with dynapenia and HGS measures than NFS in postmenopausal women. Further longitudinal studies are required to confirm the significance of our findings.
Keywords: Non-Alcoholic Fatty Liver Disease Fibrosis Score; Fibrosis; Fibrosis – 4 Score; Menopause; Handgrip Strength; Dynapenia
Abbreviations: TC: Total Cholesterol; HDL-C: High Density Lipoprotein Cholesterol; (HDL-C) LDL-C: Low-Density Lipoprotein Cholesterol; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase
Cardiovascular disease (CVD) presents the most critical risk against healthy aging and longevity [1]. The close interrelation between CVD and reproductive life is becoming more profound at the time of the menopausal transition [2]. A large amount of data has demonstrated distinct patterns of hormonal alterations and the prevalence of cardiometabolic risk factors, including dyslipidemia, hypertension and metabolic syndrome [2,3]. Nonalcoholic fatty liver disease (NAFLD), also regarded as a metabolic syndrome equivalent, is estimated to also occur rather frequently after the menopausal transition [4,5]. Nowadays, healthcare providers turn more frequently to prevention strategies to reduce the prevalence of the CV-risk components and slow down their progression [2]. In this context, the role of noninvasive indices to evaluate liver function has been considered as a cost-effective approach and can be successfully completed using simple biochemical parameters [2,3]. The fibrosis-4 (Fib-4) score and the NAFLD fibrosis score (NFS) have emerged as essential tools, which have been shown to stand out for their ability to estimate the risk for the most advanced stage of liver dysfunction, namely liver cirrhosis [6]. Furthermore, a large body of evidence has demonstrated the efficacy of Fib-4 score to effectively confirm or exclude the presence of advanced fibrosis with 89% accuracy [7]. On the other hand, the low- and high-normal cut-off values of the NFS, namely -1.455 and 0.676, are associated with a sensitivity of 90% and a specificity of 97% for advanced fibrosis, respectively. In addition, the same cut-off values have a negative likelihood ratio of 0.17 and a positive likelihood ratio of 20.3 for advanced fibrosis [8-10].
The loss of muscle strength is undoubtedly linked with aging [11]. Recent data has also highlighted that the pattern of hormone alternations at the menopause transition and the postmenopausal period is not significantly associated with low muscle strength and dynapenia [12], implying that the effect of age appears to be more detrimental than menopause per se [13]. The possible association between muscle strength and laboratory values of liver function has been investigated in adolescents [14,15] and in mixed gender middleaged populations using available NAFLD index scores [16-18]. The limited available data in women after the menopausal transition is suggesting that the postmenopausal loss of muscle strength is associated with the accumulation of features of the metabolic syndrome [19], but the possible link with indices of liver function has received limited attention, while the menopausal status was not specifically taken into consideration [16-18]. We aimed to evaluate the possible link between handgrip strength measures and values of the fibrosis-4 score and the NFS in a sample of healthy postmenopausal women.
Study Population
This cross-sectional study recruited postmenopausal women attending the outpatient Menopause Clinic of Aretaieion Hospital in Athens, Greece, from January 2021 and August 2022. The menopausal status was characterized by the absence of menstruation for at least twelve consecutive months, a serum follicle-stimulating hormone (FSH) level > 25 mIU/mL, and an estradiol (E2) level < 50 pg/mL. We excluded women with clinically overt or treated CVD, acute or chronic inflammatory diseases, diabetes mellitus of any type either diagnosed or treated, hepatic or renal insufficiency, thyroid dysfunction, history of recent surgical intervention for any reason or the presence of a tumor of any origin. Moreover, we excluded women under treatment with any of the following medications either at the time of the study recruitment or 6 months prior to this:
a) Nitrates or steroids,
b) Hormone replacement therapy or selective estrogen receptor modulators,
c) Anti-osteoporotic drugs (e.g. bisphosphonates and / or denosumab) or teriparatide. A total of 122 women fulfilling the inclusion criteria were included in the study. All participants provided informed consent, and the Local Ethics Committee approved the research protocol of the present study.
Protocol Study Procedures
A detailed medical history was recorded for all participants. We measured anthropometric parameters, including body weight (Kg) and waist circumference (cm). The body weight was measured using a digital weight scale, and the height (m) was estimated in an upright position using a stadiometer. BMI was calculated using the equation: body weight (Kg) / height (m)2. Fasting venous blood samples were collected on the same morning, centrifuged when necessary, and stored at −80 °C until assessment.
Assessment of Body Composition and Bone Status
Body composition was determined by Dual Energy X-ray absorptiometry (DXA; General Electric Lunar Corporation, Madison, Wisconsin, USA), with regional and whole-body scans, according to the manufacturer’s protocol. Body fat distribution was determined as the percentage of body fat and the absolute value of lean mass (Kg).
Physical Activity Assessment
Physical activity was evaluated using the International Physical Activity Questionnaire (IPAQ) index, which has already been validated for the Greek population, as previously described [20,21]. The IPAQ index classifies the extent of physical activity based on the amount of time spent on the following activities during the previous seven days: walking or moderate vs. high-intensity physical activity [21]. MET (metabolic equivalent of task) minutes were estimated for each category of physical activity, and the total MET-minutes per week were calculated [20,22]. Accordingly, intense physical activity was defined for those participants who met any of the following criteria:
1. ≥ 3 days of vigorous-intensity activity, achieving a minimum of at least 1,500 MET-minutes per week.
2. ≥ 7 days of combined activity, consisting of vigorousintensity or moderate-intensity activity or walking, achieving a minimum total of at least 3,000 MET-minutes per week.
3. Moderate physical activity was defined for those participants who met at least one of the following criteria:
4. ≥ 3 days of vigorous-intensity activity or ≥ 20 minutes per day.
5. ≥ 5 days of moderate-intensity activity and/or walking for ≥ 30 minutes daily.
6. ≥ 5 days of any combination of walking, moderate- or vigorous-intensity activity, achieving a minimum of ≥ 600 METminutes per week.
A sedentary lifestyle or low physical activity was defined for those participants who did not meet any of the above criteria.
Evaluation of Musculoskeletal Status
For this purpose, we used measures of hand grip strength (HGS) using a hydraulic hand dynamometer (Jamar®, Sammons Preston, Illinois, USA). The measurements were performed with the participants in a sitting position, the elbow at 90o flexion, the forearm in the neutral position and the wrist between 0 and 30° of extension. The meaning of three consecutive measurements in each hand was used for analysis (intraclass correlation coefficient 0.94-0.98 depending on the tested side) [23-25]. HGS measures below the cut off of 16 kg was considered as diagnostic of dynapenia, whereas those with higher HGS measures were considered to have normal muscle strength [26].
Biochemical and Hormonal Assays
Biochemical assays were performed on the Architect c 8000 system (Abbott Diagnostics). Serum glucose was measured by the hexokinase/G-6-PDH methodology (Abbott; coefficient of variance, CV ≤ 5%). Total cholesterol (TC) was measured by enzymatic assay (Abbot; CV ≤ 3%) and triglycerides by the enzymatic glycerol phosphate oxidase methodology (Abbott; CV ≤ 5%). High density lipoprotein cholesterol (HDL-C) levels were assessed by chromogenic accelerator selective detergent methodology (ultra-HDL assay, Abbott) (CV ≤ 4%) and low-density lipoprotein cholesterol (LDL-C) by elimination methodology (multigent direct LDL, Abbott; CV < 4%). Levels of aspartate aminotransferase (AST) was measured using the Human AST ELISA kit (Abcam), with sensitivity 43 pg/mL. The alanine aminotransferase (ALT) were measured using the human alanine aminotransferase ELISA kit (Abcam), with sensitivity of 1.875 mIU/mL. Hormonal serum assays were performed on the Architect i1000SR analyzer (Abbott Diagnostics) by chemiluminescent microparticle immunoassay (CMIA; Abbott) and included: insulin (CV ≤ 7%) and 25 (OH) Vitamin D (CV≤ 4.6%) levels. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as follows: fasting insulin (μU/mL) × fasting glucose (mmol/L)/22.5. Fib-4 score and NFS were defined using the previously defined panel equations [27,28].
Statistical Analysis
Statistical analysis was performed using SPSS version 27.0. Qualitative data are expressed as frequencies (percent values), while quantitative data are expressed as mean values and standard deviation (mean ± SD). The normality of distributions was evaluated using both exploratory data analysis and the Kolmogorov-Smirnov test. Correlations between parameters of interest were evaluated using Pearson’s correlation coefficient. The values of Fib-4 score will be evaluated as continuous variable, while values of NAFLD score will be evaluated as both continuous and dichotomous parameter. Differences between continuous variables were assessed using the independent samples t-test (for baseline observations) or one-way analysis of variance (ANOVA). Differences between dichotomous variables were assessed using Chi-square scores. Linear and logistic regression analysis were used to evaluate the potential association between Fib-4 score or NFS or dynapenia as dependent variables and indices of liver fibrosis as independent variables, adjusting for various cardiometabolic risk factors. We used variance-inflation factors to estimate co-linearity between independent variables in regression models, all factors were estimated as < 2, implying that multicollinearity did not bias the regression models. For all calculations statistical significance was set at the level of p < 0.05.
Table 1 presents the results of the descriptive analysis for the 122 postmenopausal women of our study. As per the NFS results, 58% of our women had scores indicative of fibrosis stage F0 to F2, 39% had an indeterminant score and only 3% had measures indicative of more advanced fibrosis, namely F3 to F4. Table 2 presents the results of the correlation analysis between Fibrosis 4 score, NFS and musculoskeletal as well as anthropometric/biochemical parameters. Accordingly we observed that Fib-4 score measures correlated positively with age (r-coefficient = 0.501, p-value < 0.001), menopausal age (r-coefficient = 0.373, p-value < 0.001), LDL-C (r-coefficient = -0.302, p-value = 0.002), HGS (r-coefficient = -0.213, p-value = 0.034), dynapenia (r-coefficient = 0.232, p-value = 0.020). The NFS measures correlated with age (r-coefficient = 0.421, p-value < 0.001), menopausal age (r-coefficient = 0.293, p-value = 0.003), BMI (r-coefficient = 0.361, p-value < 0.001), triglycerides (r-coefficient = 0.231, p-value = 0.022), LDL-C (r-coefficient = -0.223, p-value = 0.027), HGS (r-coefficient = -0.247, p-value = 0.015), dynapenia (r-coefficient = 0.219, p-value = 0.032).We proceeded comparing values of fibrosis-4 score between women with dynapenia and those with normal muscle strength. As shown in Figure 1a, we observed that women with dynapenia had higher values of Fib-4 compared to women with normal muscle strength, 1.34±0.6 vs 1.1±0.37, p-value = 0.016 (ANOVA). In addition, women with lower NFS cores indicative of the early stages of fibrosis had lower prevalence of dynapenia compared to women with more advanced stages of fibrosis (Fibrosis, F0-F2 vs indeterminate vs F3- F4: 13.8% vs 20% vs 66%, p-value = 0.059 chi-square value, Figure 1b).
Note: YSM=years since menopause; SBP = systolic blood pressure; DBP = diastolic blood pressure; BMI=body mass index; HDLC= high density lipoprotein cholesterol; LDL-C=low density cholesterol; HOMA-IR = homeostasis model assessment of insulin resistance. Hypertension was defined as a history of antihypertensive treatment or systolic arterial blood pressure ≥140 mmHg and/ or diastolic arterial blood pressure ≥90 mmHg measured on at least three different occasions; Hyperlipidemia was defined as a history of hypolipidemic treatment or total blood cholesterol level above 200 mg/dL or LDL-cholesterol levels higher than 160 mg/dL and/or plasma triglycerides higher than 200mg/dL; Dynapenia is defined as handgrip strength levels < 16kg.
Figure 1 a. Fibrosis-4 values between women with dynapenia and those with normal muscle strength. b. Prevalence of dynapenia according to Non-alcoholic fatty liver disease fibrosis score classification.
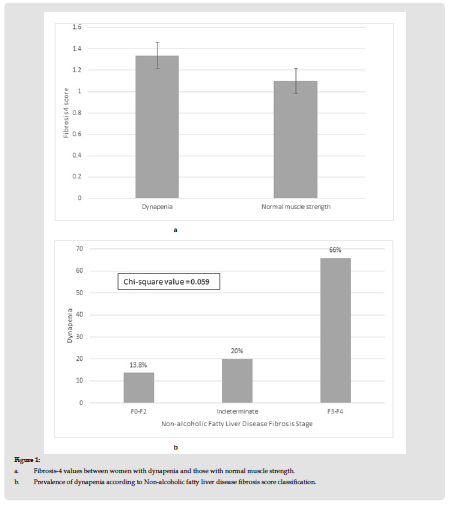
Note: Dynapenia vs Normal muscle strength, Fibrosis-4 score: 1.34±0.6 vs 1.1± 0.37, p-value 0.016 ANOVA
Dynapenia is defined as handgrip strength levels < 16kg.
Statistical analysis was set at the level of p-value < 0.05.
Table 2: Correlation analysis between Fibrosis-4 score and musculoskeletal parameters and anthropometric/biochemical parameters.
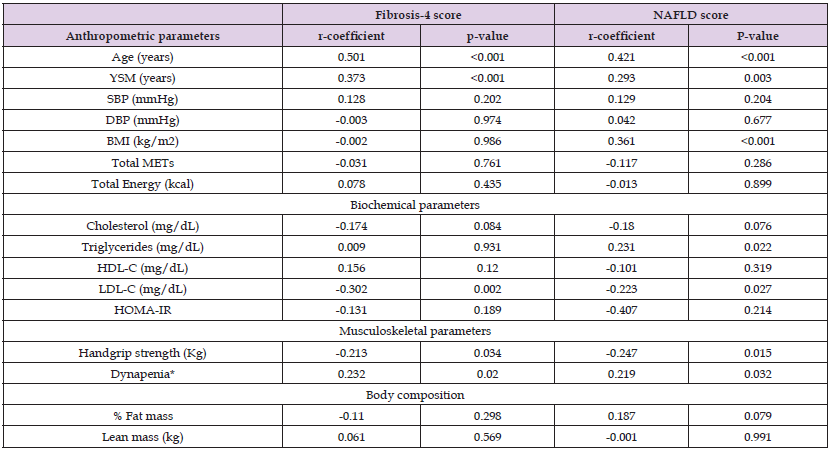
Note: YSM=years since menopause; SBP = systolic blood pressure; DBP = diastolic blood pressure; BMI=body mass index; HDL-C=high density lipoprotein cholesterol; LDL-C=low density lipoprotein cholesterol; HOMA-IR = homeostasis model assessment of insulin resistance
*Dynapenia is defined as handgrip strength levels < 16kg.
Statistical significance was set at the level of p-value < 0.05.
Table 3: Regression analysis models to evaluate the association between dynapenia (or handgrip strength) and noninvasive scores of liver function (Fibrosis 4 score or NFS).
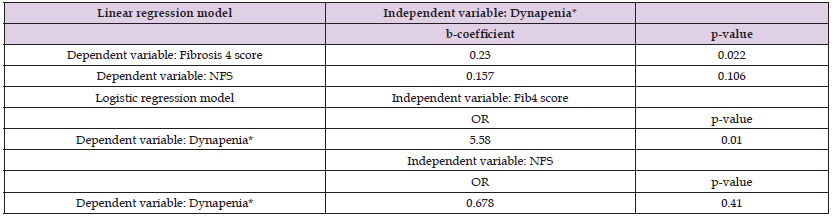
Note: Both models were adjusted for age, low-density lipoprotein cholesterol, homeostasis model assessment of insulin resistance, body mass index, total energy consumption, physical activity.
NFS=nonalcoholic fatty liver disease fibrosis score.
*Dynapenia is defined as handgrip strength levels < 16kg (reference category, normal muscle strength defined as handgrip strength≥16kg
Statistical significance was set at the level of p-value < 0.05.
Table 3 presents the results of the multivariable regression analyses. The models of linear regression analysis included Fib- 4 score or NFS as an independent variable and dynapenia as independent variable. The models of logistic regression analyses included dynapenia as dependent variable and either Fib-4 score or NFS as independent variable. All models were adjusted for age, LDL-C, HOMA-IR, BMI, total energy consumption, physical activity. We observed that Fib-4 score was significantly associated with dynapenia (b-coefficient = 0.230, p-value = 0.022), but not with NFS. Dynapenia was significantly associated with Fib-4 score (OR = 5.58, p-value = 0.010), but not with NFS.
The results of our study showed an independent negative association between HGS values and Fib-4 as well as NFS values. The values of Fib-4 score differed significantly between women with dynapenia compared to those with normal muscle strength. Based on NFS, there was a borderline association between HGS values and the severity of liver fibrosis. Multivariable analysis showed that only Fib- 4 scores were positively associated with dynapenia, which was not the case for NFS values, after adjusting for significant cardiometabolic risk factors. The possible association between measures of HGS and NAFLD indexes has been evaluated recently in various populations. An earlier cross-sectional study of 538 older adults (105 men and 432 women) described an inverse linear association between increasing HGS levels the NFS, as well as the Fib-4 score. The group of HGS values in the lowest tertile compared to the group of HGS values in the highest tertile had higher odds of a higher NFS but comparable Fib-4 score values [17]. A large study of 5272 middle-aged adults (1678 men and 3594 women), the data of which was retrieved from Korea National Health and Nutrition Examination Surveys form 2014- 2018, evaluated the cross-sectional association between low HGS (defined as values < 25th percentile) and NAFLD, defined using the comprehensive NAFLD score and the hepatic steatosis index [29]. This study showed an association between low HGS measures and higher risk of NAFLD in middle-aged adults of both gender [18]. One more nationwide study of 8001 South Korean adults (women 55.5%) described that adults with lower HGS have higher odds for NAFLD; the latter was assessed using biochemical and anthropometric parameters, based on the hepatic steatosis index [16]. These results are partly in agreement with our study of postmenopausal women; the main difference is the definition of dynapenia, which in this study was based on the latest European Guidelines on Sarcopenia [26].
Others explored the link between HGS measures and liver function in the different groups of patients. In a sample of 1270 adolescents (648 boys, 622 girls), retrieved from the cohort of the NHANES cross-sectional study, described only in boys an inverse association between low HGS measures and higher values of gamma glutamyl transpeptidase and AST, even after normalizing measures of HGS according to body composition parameters, like body weight, whole-body fat and trunk fat [15]. Similar results were reported by one more mixed-gender study in adolescents [14]. One more study reported that baseline HGS measures below 18.15 kg compared to higher measures, estimated prior to liver transplantation, represent a significant predictor of poor survival in patients with end-stage liver disease [30]. Our findings highlight a role of Fib-4 scores but not NFS values, concerning the presence of dynapenia. The association between the perimenopausal hormone alternations and the risk for NAFLD and progressive liver disease appears to be associated with accrual of cardiometabolic risk factors after the menopausal transition, as reported by our previous study [4] and by others [10,31]. Accumulation of features of the metabolic syndrome has also been linked with the postmenopausal loss of muscle strength [19]. Earlier meta-analyses highlighted the diagnostic accuracy of both Fib-4 score and NFS among non-invasive indices to monitor fibrosis in patients with NAFLD [6]. This choice is also supported by the latest guidelines to exclude advanced cirrhosis [32]. Fib-4 score in particular has been shown to represent a useful marker to exclude advanced fibrosis, even in individuals with or without steatohepatitis [33], but also in the group of NAFLD patients with normal ALT levels [34,35]. The latter subgroup of patients is estimated as the 25% of NAFLD patients and 19% of patients with non-alcoholic steatohepatitis [35].
This study has certain limitations which should be listed. First, the sample is relatively small. Second, the cross-sectional design does not permit detection of causality. Third, all women were retrieved from consecutive outpatients of a University Menopause Clinic, hence more health aware than the general postmenopausal population. Fourth, the use of Fib-4 score values is likely to overestimate fibrosis in older patients [36], given the recent data which recommended use of age-specific cut-offs. We managed to overcome this limitation by assessing Fib-4 only as a continuous variable. Fifth, Fib-4 values differ according to the ethnicity [37,38], but also this drawback was controlled by the common ethnic background of our participants, all of which are native Greek.Based on our findings, the Fib-4 score can be considered as a useful indicator of HGS measures, including the odds for dynapenia. Fib-4 score can easily be calculated, based on parameters available in primary care settings, namely the platelet count, levels of AST and ALT as well as the age [28]. This index can not only rule out advanced fibrosis [39] but also considered as a useful proxy indicator of HGS in the postmenopausal population, as per our findings. After all, lower HGS measures have been shown to indicate higher risk for cardiovascular mortality and cancer mortality [40].
In summary, this study found an inverse association between values of NAFLD indexes and HGS, in a sample of healthy postmenopausal women. Amongst the two most commonly used indexes, Fib-4 appears to be more consistently associated with dynapenia and HGS measures. If the results of this study are confirmed by longitudinal observations, this cheap and non-invasive marker will be proven as very valuable in guiding the health assessment of older individuals, including their monitoring of muscle strength.
This research is co-financed by Greece and the European Union (European Social Fund- ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning» in the context of the project “Reinforcement of Postdoctoral Researchers - 2nd Cycle” (MIS-5033021), implemented by the State Scholarships Foundation (ΙΚΥ).
All authors declare having no conflicts of interest.


