Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
István Szabó1, Tamás Molnár3, Judit Gimesiné Fodor4, Imre Biksi5, László Makrai6, László Buza7* and Tibor Magyar2
Received: February 09, 2023; Published: February 23, 2023
*Corresponding author: László Buza, Inetrvet Hungaria Kft, 1095 Budapest, Lechner Ödön fasor 8, Hungary
DOI: 10.26717/BJSTR.2023.48.007717
Respiratory disease caused by Actinobacillus pleuropneumoniae is a source of major economic loss to the swine industry all over the world. Determining the source of infection with the pathogen is an important objective of epidemiological investigations conducted in connection with disease outbreaks in pig herds. This makes it possible to establish potential responsibility in warranty issues and to tighten the biosecurity measures of pig farms. By analyzing the course of an A. pleuropneumoniae outbreak, the production processes of the farms brought into connection with the spread of infection and the lung lesions found in the pigs sent to slaughter, as well as by the detailed characterization of the pathogen cultured from the organs of animals, we determined the probable source of infection for a given farm and ruled out the role of another suspected infection source. According to our results, the infection of a fattening farm previously free from A. pleuropneumoniae originated from an infected farm located at a distance of 450 meters. The most likely way of infection spread was airborne transmission or movement of personnel between the two farms. This case highlights that the animal health requirements of future production must be taken into consideration already at the stage of designing the construction of pig farms. This includes the knowledge of the animal health status of other pig farms located in the region of the farm to be constructed, as well as the accurate determination of the ‘protective distance’ to be maintained between farms. The use of the typing scheme presented for A. A. pleuropneumoniae may allow the creation of a Hungarian and an international database which may make it possible to determine the origin of inter-herd transmission of A. pleuropneumoniae infection.
Keywords: Actinobacillus pleuropneumoniae; Epizootiology; Pneumonia; Pig
The eradication programme of porcine reproductive and respiratory syndrome (PRRS) from pig herds, approved also by the competent veterinary authorities of the European Union, has been in progress in Hungary since 2014 (Nemes, et al. [1-3]) One of the most effective, safest and fastest methods of eradication is the depopulation–repopulation method. Since the start of the programme, PRRS has been eradicated from several large pig herds by that method. In addition to eliminating PRRS infection, one of the non-negligible additional benefits of depopulation–repopulation performed in the framework of the PRRS eradication programme is enabling the replacement of the previous population with a new pig population free from other infectious diseases causing substantial economic losses. In the Hungarian practice of pig production this means that the new herd introduced after depopulation will be free not only from PRRS but also from numerous other diseases such as brucellosis, Aujeszky’s disease, leptospirosis, mycoplasma pneumonia, Actinobacillus pleuropneumoniae infection, swine dysentery, atrophic rhinitis, and sarcoptic mange, and thus will be capable of higher performance.In connection with the PRRS eradication programme, the biosecurity of pig farms has become the focus of attention. In the recent past, farms of multi-site production system where the different stages of production (nursery, prefattening and fattening) are conducted on separate sites or in separate units, have been established in increasing numbers.
This system offers biosecurity advantages over the farrow-to-finish system, which was almost exclusively used in Hungary previously. In the farrow-to-finish system all phases of the production of pigs for slaughter take place on a single site, which poses increased infection risk due to the high animal density (around 80 % of the farms are in this cathegory). It is also a consequence of PRRS eradication that since November 2017 only PRRS-free prefatteners may be introduced into the large fattening farms (the official brucellosis- and Aujeszky’s disease-free status of Hungary on nationwide level had been declared already earlier). The profitable operation of farms and the economic efficiency of fattening greatly depend on the health status of prefatteners used for slaughter pig production. Therefore, in integrated multi-site systems it is a significant economic advantage if the breeding farm can supply prefatteners free from as many diseases as possible. Among the infectious diseases, pleuritis and pneumonia caused by A. pleuropneumoniae (APP) is an important causative agent of porcine respiratory disease complex (PRDC), which causes the highest economic losses to the modern pig industry all over the world. APP can cause acute outbreaks characterised by high morbidity and mortality and substantial medication costs, while in the chronic cases it also results in huge economic losses due to reduced feed intake and body weight gain, prolongation of the fattening period and lack of uniformity within the herd. Actinobacillus pleuropneumoniae is a facultative pathogenic bacterium, and predisposing factors have a major role in the development of the characteristic disease signs.
Also in herds free from APP, strict compliance with the biosecurity measures, the soonest possible correction of all the deficiencies discovered, and accurate investigation of the causes of the possibly occurring infections are essential (Sassu, et al. [4-9]). In the epidemiological investigation of the transmission of all infectious diseases including APP, determining the origin and source of the pathogen introduced into the herd has outstanding importance. This makes it possible to establish responsibility in warranty issues and to tighten the biosecurity measures of pig farms. This paper presents a case in which the origin of A. pleuropneumoniae infection of a fattening herd free from eight important diseases (brucellosis, leptospirosis, Aujeszky’s disease, PRRS, atrophic rhinitis, mycoplasmosis, APP, swine dysentery) was studied by molecular methods in addition to the traditional epidemiological, clinical and bacteriological procedures to identify the source of the infection.
Herds Studied
Farm ‘A’:: A large farm newly constructed as a greenfield investment, which can accommodate and fatten approximately 2,000 prefatteners, and operates by strict observance of the all-in/all-out principle. Prefatteners are brought to the farm from Farm ‘D’,, the integrator’s farm of high health status free from eight important pig diseases. The farm is at an air distance of 450 metres from Farm ‘B’. The farm manager of Farm ‘B’ is among the owners of Farm ‘A’, and the same veterinarian attends to Farms ‘A’ and ‘B’. When entering the farm, these persons strictly observe the biosecurity requirements. During the investigation of pleuropneumoniae infection that occurred in Farm ‘A’ in April 2019, the suspicion arose that the causative agent of APP may have been introduced to the farm from Farm ‘B’ or Farm ‘C’.
Farm ‘B’: A farrow-to-finish herd of almost 900 sows, in which pleuropneumoniae occurs regularly and the clinical manifestations of infection are treated successfully with antibiotics.
Farm ‘C’: A fattening farm with 15,000 fattening places and continuous replenishment, in which the pigs are fattened in 20 houses with 300–1,800 fattening places each. In 2018 the farm was stocked exclusively with pleuropneumoniae-infected prefatteners originating from Germany. Only the chronic clinical form of APP occurred. From the beginning of 2019, the all-in/all-out principle was introduced on building level and the farm have been stocked with prefatteners originating from Farm ‘D’, as the stock introduced to Farm ‘A’. This farm is attended to by the same veterinarian who supervises compliance with the animal health provisions in Farm ‘A’ as well.
Farm ‘D’: a large farrow-to-prefattening type pig farm, which is also free from the eight major infectious diseases listed above. This is the farm from which the integrator supplies prefatteners to both self-owned and contract fattening farms.
To Farm ‘A’, prefatteners were introduced in February 2019. The tests performed at that time demonstrated the A. pleuropneumoniae, Pasteurella multocida and Brachyspira hyodysenteriae free status of the herd. Sixty-four days after stocking, an acute APP manifested in clinical signs occurred. The laboratory tests demonstrated the presence of biotype 1, serotype 2 A. pleuropneumoniae in the pigs having died of the disease.
On Farms ‘A’ and ‘C’ we studied the pathogenesis of the outbreak, the appearance of clinical signs, the pathological lesions, the mortality occurring during the fattening phase and the percentage proportion of the pigs sold for slaughter. At the end of May 2019, we took samples at the slaughterhouse from pigs originating from the three affected herds (‘A’, ‘B’ and ‘C’). That study involved those fattening pigs of Farm ‘A’ that were finished last. The pigs slaughtered from Farm ‘C’ represented the fatteners introduced from Farm ‘D’ as prefatteners in January. The pigs tested from Farm ‘B’ originated from the continuous production of pigs for slaughter.
Laboratory Tests
Laboratory tests were conducted from the herd of Farms ‘A’, ‘B’ and ‘C’, partly from dead pigs collected at the farms, and partly from lung samples collected during slaughterhouse investigations. Bacterial culture was performed from organs with pathological lesions. To characterise the A. pleuropneumoniae isolates, we determined their biotype and serotype, conducted a toxin–lipoprotein multiplex PCR and sequenced the 950-bp DNA segment targeting the omlA outer membrane lipoprotein gene. The antibiotic susceptibility profile of the strains was also determined. The isolation and biotype determination of A. pleuropneumoniae was performed on a culture medium supplemented with 5% sheep blood (Columbia agar, LAB M) incubated at 37°C in 5% CO2 atmosphere for 48 h. On that medium, biotype 2 strains showed abundant growth, while the biotype 1 strains (requiring NAD) could be cultured only as satellite colonies in the presence of Staphylococcus aureus.
Molecular species identification was performed as described by (Gram, et al. [10]). The isolates were classified as A. pleuropneumoniae species based upon the detection of a species-specific 950-bp gene segment targeting the omlA outer membrane lipoprotein gene. The PCR products obtained during the species-specific PCR were sequenced. Sequencing of PCR products was performed by Macrogen Europe (Amsterdam, The Netherlands). Nucleotide sequences were aligned and compared using Geneious Prime software (version 2019.2.1).
Nucleotide sequence data were analysed using MEGA7 software (Kumar, et al. [11]). By analysing the obtained sequences, we constructed a phylogenetic tree that illustrates the differences between the omlA genes of the different strains. The toxin–lipoprotein multiplex PCR was performed as described by (Gram, et al. [12]). By studying the apx and omlA gene segments of A. pleuropneumoniae, we determined the profiles typical of the different strains. For the reactions we used the primers targeting the structural genes of the apx toxins (apxI, apxII, apxIII) and their secretory (transport) genes (XIBD, XIIIBD). In the reactions targeting the omlA gene segment, we could classify the strains into different groups (1–4) on the basis of differences occurring in the omlA sequence. The antibiotic susceptibility of the strains was determined by the disk diffusion method as described by (Sárközi, et al. [13]) and (Sárközi [14]), and the developing inhibition zones were interpreted by following the guidelines provided in the CLSI VET01-S2 document (CLSI [15-16]).
Disease Course of APP in Farm ‘A’
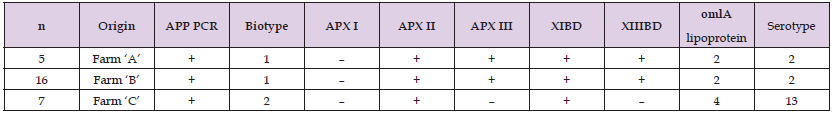
In February 2019 the newly constructed farm was stocked with 1,900 prefatteners originating from Farm ‘D’ free from the 8 major infectious diseases listed above. The serological tests and nasal swab bacterial culture for A. pleuropneumoniae, performed on day 3 after arrival in Farm ‘A’ to demonstrate A. pleuropneumoniae free status gave negative results. During the 61 days after introduction, a total of 14 deaths (0.7%) occurred in the herd. By on-the-spot necropsy of the dead pigs the attending veterinarian found rectal prolapse, leg problems, fibrinous pericarditis, liver degeneration, chronic enteritis of the small intestine, chronic oesophagogastric ulcer, and internal bleeding into the gastrointestinal tract. On day 62 after introduction into the farm, three dead pigs were diagnosed with croupous pneumonia and fibrinous pleuritis during necropsy performed on the spot. Because of the suspected involvement of A. pleuropneumoniae, antibiotic therapy (amoxicillin administered via the drinking water and, in the case of animals showing severe clinical signs, by parenteral route) was given. Two days later the number of disease cases substantially increased and there was a large increase in the number of deaths (to 15 pigs) as well (Figure 1). Gross pathological examination of the organs of three dead pigs submitted to the laboratory revealed acute fibrinous-haemorrhagic-necrotic pleuropneumonia and bacterial culture from the affected lung areas yielded biotype 1 and serotype 2 A. pleuropneumoniae. The strains harboured the structural genes of apx toxins APXII and APXIII, with their secretory genes XIBD and XIIIBD.
The strains possessed omlA lipoprotein gene type 2, which was confirmed by sequencing (Table 1). The antibiotic susceptibility test results of the isolate are presented in (Table 2).Of the 1,900 prefatteners introduced to the farm, 1,793 (90.1%) were subjected to full-value slaughter after the 108-day fattening period, 91 pigs (4.6%) were subjected to a so called technological slaughtering (lower waight), while 106 animals (5.3%) died during the finishing phase.
Figure 1 Number of Deaths Occurring in Farm ‘A’ from the Arrival of Prefatteners up to the Time of Sending to Slaughter.
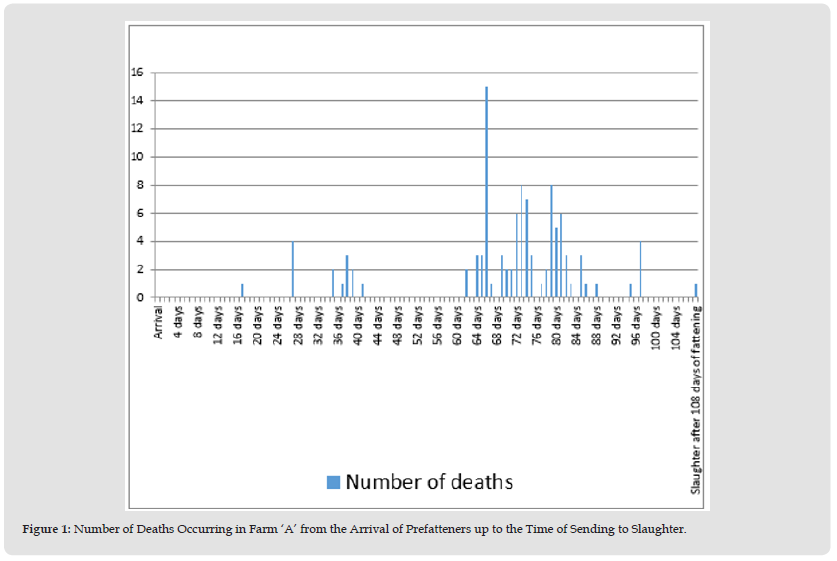
Table 1: Properties of the Actinobacillus Pleuropneumoniae Strains Isolated During the Slaughterhouse Inspection.
Disease Course of APP in Farm ‘C’
In 2018, a prefattener stock infected with A. pleuropneumoniae arrived in the farm from Germany. In January 2019, by the laboratory testing of lung samples from three pigs in the herd biotype 2 and serotype 13 A. pleuropneumoniae was detected in two cases, while in the third case a biotype 1 A. pleuropneumoniae giving equal reaction with sera specific for serotypes 6 and 8 was demonstrated. From January 2019, a prefattener stock free from the above-specified 8 major infectious diseases was brought into the same farm from Farm ‘D’, always into previously depopulated and disinfected separate buildings. All this meant that between January 2019 and late April 2019, on the farm there were simultaneously buildings that contained A. pleuropneumoniae-infected pigs originating from Germany, although in a decreasing number, as well as buildings in which A. pleuropneumoniae-free stocks were kept (the number of the latter buildings was increasing). From the end of April 2019, all buildings of the entire farm contained only pigs originating from the stock free from the 8 important infectious diseases. The analysis of farm data (Figure 2) clearly shows that in 2018 the mortality rate continuously increased and then remained on a high level. This was typical also for the simultaneous keeping of the two herds of different origin and A. pleuropneumoniae infection status. Then, as the herd free from the 8 major infectious diseases became predominant, the number of deaths markedly decreased.
During the laboratory tests performed in Farm ‘C’ simultaneously with the introduction of infection to Farm ‘A’, by the examination of the organs of 10 pigs that died on 16 April and 25 April 2019, A. pleuropneumoniae was detected in three cases: two strains belonged to biotype 2 and one to biotype 1. The strains harboured the structural genes of apx toxins APXII and APXIII, with their secretory genes XIBD and XIIIBD. The strains possessed omlA lipoprotein gene type 2, which was confirmed by sequencing (Table 2). Out of the 34,250 prefatteners originating from Germany and stocked into Farm ‘C’ between May and December 2018, after a fattening period of 109–151 days 32,201 pigs (94%) were slaughtered in full value, 1,109 pigs (3.2%) were subjected to technological slaughter, and 940 pigs (2.7%) died during the fattening. Out of the 17,600 prefatteners introduced between January and May 2019 from the ‘8 diseases free’ herd of Farm ‘D’, 16,084 finishing pigs (91.4%) were slaughtered in full value, 809 pigs (4.6%) were subjected to technological slaughter and 707 pigs (4.0%) died during the fattening period. On that farm, the fattening results of the first two batches introduced in January 2019 were expressly unfavourable, and the ratio of pigs slaughtered in full value to all prefatteners introduced was only between 86.5 and 86.6%, while for the prefattener batches stocked in in March and April this ratio was already above 95%. A few days after its arrival, the prefattener stock brought in from Farm ‘C’ in May 2019 was subjected to serological testing which confirmed the A. pleuropneumoniae free status of the herd of origin.
In summary, it can be established that the herd in Farm ‘A’ was originally A. pleuropneumoniae free but became newly infected 60 days after introduction, and the subsequent addition of pigs from an A. pleuropneumoniae free herd resulted in the appearance of the clinical signs of infection and a marked increase of the mortality rate. In the case of the chronically infected herd in Farm ‘C’, the relatively stabilised A. pleuropneumoniae infection status was disturbed by the addition of pigs originating from an A. pleuropneumoniae free herd, which also led to the appearance of the clinical signs of infection and a substantial increase of related mortality.
Testing of the Pig Herd of Farm ‘B’ for Pleuropneumoniae Infection
In April 2019, during the laboratory testing of infection in Farm ‘A’, simultaneously with the testing of the other two farms, the A. pleuropneumoniae strains cultured from the organs of four pigs sent to slaughter from the herd of Farm ‘B’ belonged to biotype 1 and serotype 2. The strains harboured the structural genes of apx toxin APXII, with their secretory gene XIBD. The strains possessed omlA lipoprotein gene type 4, which was confirmed by sequencing (Table 2).The antibiotic susceptibility test results of strains cultured from the different herds indicate that the antibiotic susceptibility of A. pleuropneumoniae strains isolated in Farm ‘A’ was fully identical with that of A. pleuropneumoniae strains detected in Farm ‘B’ (Table 1). The antibiotic susceptibility profiles of the biotype 1 A. pleuropneumoniae strain from Farm ‘C’ and of the biotype 2 A. pleuropneumoniae strain detected from the same farm differed from the antibiotic susceptibility profiles of both the Farm ‘A’ strains and from each other with regard to numerous active substances (Table 1). The altogether 55 samples collected at the slaughterhouse (23, 21 and 11 samples from Farms ‘A’, ‘B’ and ‘C’, respectively) were transported to the testing laboratory immediately after collection (within 3–5 hours). In the laboratory, A. pleuropneumoniae was successfully cultured from 6 out of 23 samples from Farm ‘A’, 16 out of 21 samples from Farm ‘B’ and 7 out of 11 samples from Farm ‘C’ (Table 2). The 7 strains isolated from lungs collected at the slaughterhouse from Farm ‘C’ and the 3 strains previously isolated from Farm ‘C’ in a different laboratory could be distinguished from strains occurring in both Farms ‘A’ and ‘B’ with full certainty.
Note: S = Susceptible, R = Resistant, I = Intermediate, – = Not Tested.
The 16 strains isolated at the slaughterhouse from lung lesions of pigs from Farm ‘B’ and the 2 strains previously isolated in a different laboratory showed similar characteristics. The 5 strains isolated at the slaughterhouse from pigs from Farm ‘A’ and the 2 strains isolated from pigs of the same farm in a different laboratory showed similar characteristics with each other and with the strains from Farm ‘B’ in all tested properties, and thus their common origin can be considered highly probable. By studying the epizootiological features of A. pleuropneumoniae infection occurring in the three farms (disease course, evolution of the mortality rate, production results, method of prefattener introduction, external and internal biosecurity) and by the laboratory examination of dead animals and pigs sent to slaughter (gross pathological examination, bacterial culturing, traditional and molecular microbiological typing, serology) we established the following:
Farm ‘A’ was stocked with a prefattener stock free from A. pleuropneumoniae, as confirmed also by the results of laboratory tests. Farm ‘C’ could not be the source of infection for the herd kept in Farm ‘A’. The herd of Farm ‘A’ most likely became infected with the A. pleuropneumoniae strain present in the herd of Farm ‘B’. The infection of Farm ‘A’ could occur either as a result of airborne transmission (facilitated by the small distance between the two farms) or could be associated with the non-observance of farm biosecurity rules applying to personnel or the objects used by them.
By analysing the course of an A. pleuropneumoniae outbreak, the production processes of the farms brought into connection with the spread of infection and the lung lesions found in the pigs sent to slaughter, as well as by the antibiotic susceptibility testing and molecular study of the pathogen cultured from the organs of dead and slaughtered animals, we determined the origin of infection for a given farm and ruled out the role of another suspected infection source. On Farm ‘A’, the infection was caused by an A. pleuropneumoniae biotype 1, serotype 2 strain, and the same strain occurred also in the herd of Farm ‘B’, causing chronic infection. The herd in Farm ‘C’ was infected by two different A. pleuropneumoniae strains at the same time: biotype 2, serotype 13 and a biotype 1, serotype 6–8 A. pleuropneumoniae strains could be detected simultaneously.
We determined with high likelihood that the infection of a fattening farm free of A. pleuropneumoniae originated from the infected herd of a farm located at a distance of 450 metres. The most likely way of infection spread was airborne transmission or movement of personnel between the two farms. This case highlights that the animal health requirements of future production must be taken into consideration already at the stage of designing the construction of pig farms. This includes the knowledge of the animal health status of other pig farms located in the vicinity of the farm to be constructed, as well as the accurate determination of the ‘protective distance’ to be maintained between farms. Also, it is very important to ensure that the professionals (farm manager, attending veterinarian) employed at a given farm are not shared between farms.
The use of the typing scheme presented for A. pleuropneumoniae may allow the creation of a Hungarian and an international database which may make it possible to determine the origin of the inter-herd transmission of A. pleuropneumoniae infection, potentially facilitating the investigation of warranty issues.
The undersigned László Búza DVM, PhD declares, that none of the tests described in the manuscript, Demonstrating the interherd transmission of Actinobacillus pleuropneumoniae infection by epidemiological and molecular methods of pathogens-2113089 required the Ethics Committee or Institutional Review Board approval. There were no any experimental animal being involved into the study.
This statements are confirmed all of my co-authors to me.


