Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Guiyun Yi1,3-5*, Xikui Wang1,3,4, Zhengting Zhang1,3,4 and Xiaodong Wang2*
Received: February 11, 2023; Published: February 22, 2023
*Corresponding author: Guiyun Yi, College of Chemistry and Chemical Engineering, Henan Polytechnic University, Collaborative Innovation Center of Coal Work Safety, Henan Key Laboratory of Coal Green Conversion, Program for Innovative Research Team in the University of Henan Province (21IRTSTHN006), Jiaozuo 454003, China
Xiaodong Wang, Department of Materials Science and Engineering, Henan Polytechnic University, Jiaozuo 454003, China
DOI: 10.26717/BJSTR.2023.48.007712
An improved self-assembly mechanism of polystyrene (PS) colloidal microspheres at the water-air interface is proposed and studied systematically. We conclude that to achieve the multi-layer selfassembly, it should satisfy the following two requirements: 1) the density difference between PS microspheres and the dispersion medium should be small enough to enable the PS microspheres to reach the water-air interface easily; 2) The wettability of PS microspheres assembled at the waterair interface changes from wetting to non-wetting with the evaporation of dispersion medium, which allows the water-air interface to stay at the bottom of the assembly body for the continuous self-assembly process. To confirm our conclusion, experiments in combination with theoretical calculations are offered in this article.
Keywords: Self-Assembly; PS Colloidal Crystals; The Water-Air Interface; Density Difference; Wettability
Colloidal crystals assembled by submicron polystyrene (PS) microspheres, a new type of three-dimensionally (3D) ordered submicron material, have attracted significant attention among the researchers due to their potential application in a variety of technologic fields, spanning over, photonic crystals, [1,2] optical filters, [3] optical switches, [4] and chemical and biochemical sensors [5]. In general, fabrication of a 3D colloidal crystal is achieved by stacking the monodispersed colloids into an ordered opal structure. Heretofore, various self-assembly techniques have already been developed for fabrication of colloidal crystals [6-9]. For example, Wang et al. successfully assembled the polymer colloids into ordered colloidal crystals on flat surfaces and the strong attractive capillary forces at a meniscus accounted for the formation of 2D or 3D crystal structure [10-14]. Szekeres and his coauthors added ionic surfactants into the suspension to adsorb the charged colloidal particles with the ionic ends of the surfactants by electrostatic attraction [15].
Furthermore, the hydrophobic end of the surfactant oriented outwards making the colloidal particles and the dispersion medium non-wetting, thus the highly ordered monolayer film composed of colloidal particles were formed at the water-air interface. However, fabrication of colloidal crystals by this method is limited to colloids with particle diameters smaller than 400 nm, because for larger colloidal particles the gravity sedimentation rate outweighs the liquid level falling rate caused by solvent evaporation. This resulted in the assembly process failed to continue. Cusola’s group has solved the problem of particle sedimentation (~800 nm) during crystallization at the meniscus between the vertical substrate and colloidal sol by increasing the temperature gradient of the suspension [16-18]. This temperature gradient induces a convection flow, which can prevent sedimentation and simultaneously offer a continuous particle flow towards the meniscus region [19]. Proposed a novel technique for three-dimensional self-assembly of PS colloids at a water-air interface.
In this work, the assembly mechanism of PS colloidal crystals was investigated and analyzed. As a result of water evaporation, the convection flow of the dispersion medium will drive the PS microspheres to the water surface. Subsequently, the strong capillary forces at the meniscus caused the PS microspheres to assemble into an ordered sequence and the effective density of the colloidal crystals was lower than the density of water, provoking them into floating on the surface of the suspension. However, this mechanism can only explain the successful assembly of the first layer,but fail to demonstrate the formation of the second layer as well as the multiple layers. Based on the research of Park et al., this study conducts a further investigation as well as an in-depth analysis and gives a new interpretation of the multi-layer assembly process of PS microspheres at the water-air interface.
Preparation of PS Colloidal Microspheres
The first step involved the pretreatment of styrene monomer in order to obtain the purified styrene. A typical procedure was performed in a separatory funnel (500 mL) and sodium hydroxide (5 wt%) was then added into the styrene solution subsequently shaking the mixed solution in order to remove the polymerization inhibitor recognized as a phenolic compound, which was called extraction separation. The above operation procedures were repeated three times. The solution treated by extraction separation was then placed into the distilling flask for further purification through decompressing distillation and eventually obtained the purified styrene. PS colloidal microspheres with sub-micrometer sizes for fabricating photonic crystals were synthesized by a traditional method of emulsion polymerization. Deionized water was poured into the jacket reactor, temperature kept at 80 ℃ and stirred at 350 rpm. Sodium styrene sulfonate as emulsifier and sodium hydrogen carbonate as buffer were added into the water. Then certain amount of styrene monomer was added into the solution 10 min later. After 1 h, potassium persulfate as initiator was introduced into the mixed solution. Finally, the polymerization was performed for 18 h to obtain the milk-white product.
Self-Assembly of PS Colloidal Crystals
The suspension of PS microspheres with a mass fraction of 9.34 wt% was placed in a beaker and treated in an ultrasonic oscillator for three times, 3 min each. Subsequently, the water in the beaker was completely evaporated after heating for 6~8 h in a 95 ℃ thermostat and PS colloidal crystals were finally obtained.
Measurement of Contact Angles
The contact Angle of the dispersion medium on the PS colloidal crystal surface was measured by JZ95A image analyzer. Since the contact angle of droplet on solid surface is influenced by droplet volume, ambient temperature and test time, which will bring errors to the measurement, the experiment in the present study was carried out at room temperature (25 ℃), the droplets were controlled at 1 L, and the wetting angle was measured in 30 s. In addition, the magnification of the objective lens was 4 X while that of the eyepiece was 17 X.
Figure 1 SEM images of the top surface and cross section of the PS colloidal crystals, respectively.
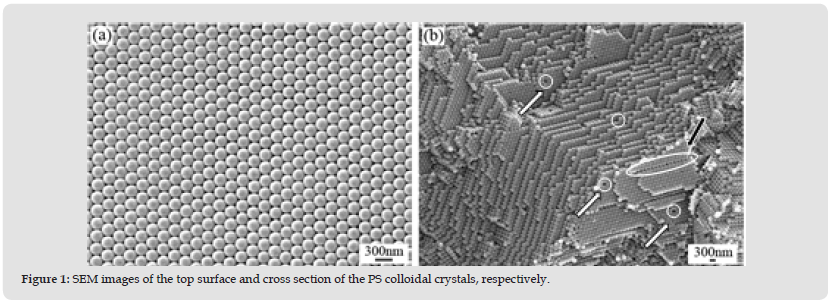
Shown in (Figure 1) are the SEM images of PS colloidal crystals prepared by evaporation-induced self-assembly approach. (Figure 1a) shows the morphology of the upper surface of PS colloidal crystals, from which we can observe that the PS colloidal crystals are arranged in the form of face-centered cubic (fcc) structure. The microspheres on the upper surface are in a highly ordered arrangement and the ordered region of the crystal can reach up to at least 50×50 m2. As can be seen in (Figure 1b), the PS microspheres in the inner part of the crystal also arrange orderly. Moreover, defects, such as hole defects (marked by the white arrow in (Figure 1 b) and dislocation defects (marked by the black arrow in (Figure 1b), can also be observed from the SEM image of the cross section of the crystal. The colored luster appeared in a specific area on the surface of the suspension during an early heating period, which is a result of the occurrence of Bragg diffraction in visible light induced by the ordered arrangement of PS colloidal crystals. Therefore, the luster was a simple criterion for the ordered arrangement of colloidal particles. As the assembly process continued, the partial area with colored luster constantly expanded along the horizontal plane until it covered the whole surface of the suspension. After that, the first layer of the assembly was formed and its surface area was no longer enlarged while the assembly gradually thickened to form multiple layers with the evaporation of the dispersion medium.
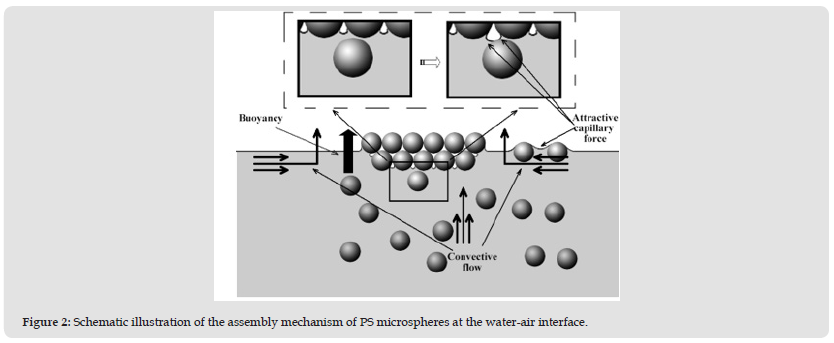
The mechanism by which the PS colloidal microspheres selfassemble into a 3D ordered structure at a water-air interface is subjected to in-depth investigation and illustrated schematically in (Figure 2). Park et al. proposed that a successful assembly at the water-air interface should satisfy two conditions:
Figure 2 Schematic illustration of the assembly mechanism of PS microspheres at the water-air interface.
The apparent density of PS colloidal crystal, which refers to the mass of the assembly per unit volume including the volume of both PS microspheres and interspherical pores, should be less than the density of dispersion medium.
The density difference between the PS microspheres and the dispersion medium should be small enough to allow the PS colloidal crystal to float on the surface of the suspension during the evaporation process.
This mechanism is a good explanation for the successful assembly of the first layer, but unable to explain the multilayer assembly process. For second layer, although the corresponding PS microspheres can be transported to the bottom of the first layer by the convection flow, they fail to self-assemble into ordered colloidal crystals due to the lack of strong attractive capillary forces. This is because they are unable to reach the gas-liquid interface covered by the upper colloidal crystals in the first layer. Therefore, a reasonable explanation for the multilayer assembly process is required and the concept of transition wettability of the PS microspheres is thus proposed. The PS microspheres prepared by emulsion polymerization can be stable in water for a long time, indicating that the spheres are completely wetted with water, and the contact angle should be close to 0°. Shown in (Figure 3) is the measured contact angle (θ) between dried PS colloidal crystals and water, which equals 112°. Furthermore, the wettability of a solid surface is related to the surface roughness. Because the dried PS colloidal crystal surface is regularly rough rather than flat, the quantitative relationship between θ ‘ (PS microspheres and water) and θ (dried PS colloidal crystals and water) can be obtained by Wenzel equation: [20,21].
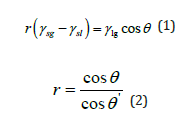
Where r represents the roughness degree of dried PS colloidal crystals; γsg, γsl, and γlg denote the surface tension between solid and gas, solid and liquid, and liquid and gas, respectively. The surface roughness of PS colloidal crystals (r) is greater than 1. Considering that the surface of PS colloidal crystals can be regarded as a rough surface composed of multiple hemispherical spheres with the same diameter, the roughness (r) can be calculated by the following equation:
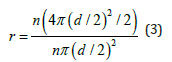
Where n and d represent the number and diameter of the PS spheres, respectively. Considering that part of the water in the droplet on the surface of colloidal crystals is in contact with the air in the pores, the actual value of r is less than the calculated value that equals 2 in Equation (3). Therefore, r is located between 1 and 2 and the value of θ ‘ ranges from 90° to 112°, which proves that the PS microspheres are non-wetted. Such transition wettability of PS microspheres may be ascribed to the different dissociation states of polar groups (including surfactants) on the surface. Based on the results presented above a novel assembly mechanism is proposed here after systematic investigation, as illustrated in (Figure 2). The convective flow transports the PS microspheres to the water-air interface and the strong attractive capillary forces drive the microspheres to approach to each other and assemble into ordered regions.
Simultaneously, the evaporation of dispersion medium causes a temporary moisture loss in the pores of the assembly and the wettability of the PS microspheres at the water-air interface changes from complete wetting to partial wetting and finally to non-wetting. Due to the change of wettability, the dispersion medium cannot be replenished into the pores and the liquid level of the suspension gradually drops below the first layer. As a result, there will still be a meniscus between the transferred PS microspheres and the spheres in the first layer and the attractive capillary forces formed will pull the PS microspheres together to assemble with the first layer, as shown in the enlarged image in (Figure 2). Multiple layers are formed by a repetition of this process. Therefore, the transition wettability plays a crucial role in achieving multilayer self-assembly at the water-air interface. To confirm the pivotal role of transition wettability in the assembly process at the water-air interface, comparative experiments are also carried out.
A series of PS suspensions containing different proportions of ethanol-water as the dispersion medium were prepared. PS suspension with pure water as the dispersion medium (system I) and PS suspensions containing different proportions of ethanol-water (system II) were heated at 90 ℃ at the same time and the assembly phenomenon at the interface was observed carefully. For system I, the obvious interface phenomenon was discovered in a relatively short period (about 10 min) and the assembly process continued until the end of the assembly. However, obviously different from system I, it took system II a longer time to form the first layer assembly and the growth rate of the crystals is slow. In addition, within the same time, the surface area of the assembly decreased with the increase of the ethanol mass fraction in the dispersion medium and when the ethanol mass fraction reached or exceeded 28%, assembly at the interface no longer occurred. In the system without interface assembly, assembly will not be found until the dispersion medium is evaporated to a large extent. The distinctive difference of interface assembly in different systems obviously correlates with the different wettability between PS colloidal crystals and dispersion medium. To prove this perspective, the contact angles between dried PS colloidal crystals and the alcohol-water mixed dispersion medium in system I were measured.
Figure 4 The contact angle between dried PS colloidal crystals and alcohol-water mixed dispersion medium varies with the alcohol mass fraction.
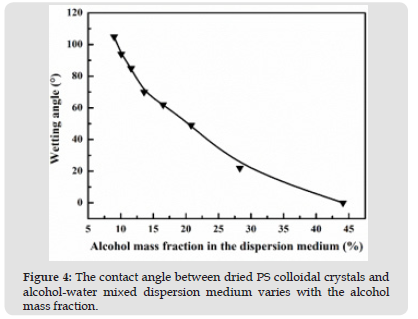
The relationship curve of the measured contact angle (θ) between dried PS colloidal crystals and the alcohol-water mixed dispersion medium in system II is revealed in (Figure 4). According to Wenzel formula, wettability of wetted surfaces can be improved after coarsening while that of non-wetted surfaces can be decreased after coarsening. Therefore, the contact angle between PS microspheres and water (θ’) is bounded by 90° and it decreased with the increase of ethanol mass fraction. When the mass fraction of ethanol is less than 10.7%, the contact angle (θ’) exceeds 90°, indicating that they are non-wetting. However, when the mass fraction exceeds 10.7%, the contact angle (θ’) is smaller than 90° and the PS microspheres become wetting with a positive correlation between the wettability and the ethanol mass fraction. In the experiment, no interface assembly phenomenon was observed with the corresponding contact angle (θ) being 22°.
According to roughness (r) and formula (2), θ’ is located between 22° and 62°. The contact angle between PS microspheres and water when no assembly occurs is designated as critical contact angle (θ’critical). On the other hand, PS colloidal crystals are usually arranged in the form of a fcc structure, as shown in (Figure 1), where PS microspheres account for 74% in volume and 26% for porosity. Hence, the calculated apparent density of PS colloidal crystals is about 0.78 g/cm3 (the density of PS is about 1.05 g/cm3 and that of air is about 0 g/cm3). The PS suspension without assembly possesses an ethanol mass fraction of 28% and a density of about 0.93 g/cm3 (the density of ethanol is 0.79 g/cm3 and that of water is 1 g/cm3). As the apparent density of PS colloidal crystals (0.78 g/cm3) is lower than that of mixed dispersion media (0.93 g/cm3), according to the conclusion of Park et al., an assembly should be observed, but in fact no interface assembly appears.
The above experimental results indicate that the apparent density of PS colloidal crystals lower than the density of dispersion medium is only one of the factors for successful assembly at the water-air interface. Meanwhile, the contact angle between PS microspheres and the dispersion medium must exceed a critical value (θ’critical). When the contact angle is less than θ’critical, even if a few PS microspheres can be temporarily assembled, the dispersion medium in their pores will still be continuously supplemented when it is evaporated. Thus, the apparent density of PS colloidal crystals filled with dispersion medium in the pores outweighs the density of dispersion medium, which causes the instantaneously formed assembly to sink into the suspension and unable to continue the assembly process. The interface assembly of PS suspension with 28% ethanol mass fraction can be observed in the late period, which may be attributed to the evaporation of ethanol in the mixed dispersion medium. As the ethanol ratio in the dispersion medium decreases, the contact angle between PS microspheres and the dispersion medium increases with the worse wettability at the gas-liquid interface and assembly occurs when the contact angle is greater than θ’critical. As a result, this experiment simultaneously confirms that the transition wettability of PS microspheres plays a crucial role in the continuous assembly process at the water-air interface.
In summary, evaporation-induced self-assembly of PS microspheres is carried out at the water-air interface and the assembly mechanism is subjected to re-investigation based on Park et al.’ work. An improved mechanism is obtained and to achieve the multi-layer assembly, it should satisfy the following two requirements:
The density difference between PS microspheres and the dispersion medium should be small enough.
The wettability of PS microspheres changes with the evaporation of dispersion medium and finally becomes non-wetting.
The apparent density of PS colloidal crystals will be smaller than that of the dispersion medium so that the assembly body can float on the surface of the suspension for continuous assembly process.
The authors declare no competing financial interests.
This work was supported by the National Natural Science Foundation of China (51974110, U1803114), the Fundamental Research Funds for the Universities of Henan Province (NSFRF180313), the Education Department Science Foundation of Henan Province (19A440002, 19A530002), the Key Scientific and Technological Project of Henan Province (202102210183) and the Young Key Teacher Training Foundation of Henan Province’s Universities (2017GGJS052).


