Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Zeiad A AlObead1, Monira AlNasser1, Asem AlMesfer1*, Sarah Alkhezzi1, Ghada Alhayaza2, Rasha Alhamazani1, Aseel Alfahhad1, Bushra Saeed Alasmari1, Mohammed Aljughayman1 and Faris A Alhomida1
Received: February 06, 2023; Published: February 20, 2023
*Corresponding author: Asem AlMesfer, Department of Dermatology, King Fahad Medical City, Prince Abdulaziz Ibn Musaid Ibn Jalawi St, Riyadh 12231, Saudi Arabia
DOI: 10.26717/BJSTR.2023.48.007701
Introduction: Cardiofaciocutaneous syndrome (CFC) type 4 is a rare, phenotypically diverse, autosomal dominant, multiple congenital anomaly disorder, that is characterized by a distinctive set of dysmorphic craniofacial features, congenital heart disease, and cutaneous abnormalities. It is caused by mutations along the Ras/MAPK cell signaling pathway, which has been extensively studied for its role in oncogenesis.
Case Presentation: A 13-year-old boy with a history of macrocephaly, polyneuropathy, cataracts, myopia and palmoplantar hyperhidrosis presents with an eruption of multiple benign melanocytic nevi. Genetic testing and parental segregation analysis confirmed de novo sporadic mutation of MAP2K2 (Mitogen- Activated Protein Kinase Kinase 2) gene of chromosome 19p13.3, confirming the diagnosis of CFC type 4.
Discussion: CFC type 4 is considered one of the RASopathies, along with Noonan syndrome and others. RASopathies are known to have mutations along the Ras/MAPK cell signaling pathway, which has been extensively studied for its role in oncogenesis and has been implicated in the pathogenesis of melanomas, ovarian and lung cancers. Given the phenotypic variation and clinical overlap of these RASopathies, genetic testing is needed to confirm the diagnosis.
Conclusion: Consider genetic testing, a multidisciplinary approach, patient education and regular skin checks in a patient that presents with a constellation of findings in association with an eruption of multiple pigmented lesions on the skin.
Abbreviations: CFC: Cardiofaciocutaneous; MEK2: Mitogen-Activated Protein Kinase Kinase 2; CBC: Complete Blood Count; CMP: Comprehensive Metabolic Profile, ESR: Erythrocyte Sedimentation Rate (ESR), ANA: Antinuclear Antibody, RF: Rheumatoid Factor, CK: Creatine Kinase, TET: Thyroid Function Test
Cardiofaciocutaneous syndrome (CFC) type 4 is a rare, autosomal dominant, multiple congenital anomaly disorder that is caused by mutations in the MAP2K2 (Mitogen-Activated Protein Kinase Kinase 2, also known as MEK2) gene of chromosome 19p13.3. Cases are often reported to be secondary to sporadic de novo deletions. Similarly, to Noonan Syndrome, and others, it’s considered one the RASopathies, with germline mutations along the Ras/MAPK cell signaling pathway. This signaling pathway is involved in the regulation of cell differentiation, proliferation, migration, and apoptosis [1-5]. Its phenotypic diversity can take on a multitude of forms, with patients exhibiting a subset or all of the traits; hence, not all of the physical findings must be present for a diagnosis. CFC is characterized by a distinctive set of dysmorphic craniofacial features, congenital heart disease and ectodermal dysplasia or cutaneous abnormalities. Multiple systems, including the ophthalmic, neurological, musculoskeletal, gastrointestinal, urinary, endocrine, and lymphatic, may also be affected. In addition, growth retardation and failure to thrive have also been reported [1-2].
Cutaneous manifestations are broadened in particular to include a myriad of findings including curly hair, hair abnormalities including sparse eyelashes, eyebrows and hair. They may present with xerosis, or have keratinization disorders such as, palmoplantar hyperkeratosis, keratosis pilaris and ulerythema ophryogenes; which is characterized by perifollicular erythematous papules in association with scarring and alopecia. Patients may also have a history of atopic dermatitis, hyperhidrosis, ichthyosis or infantile hemangiomas [1,6]. The exact prevalence of CFC is unknown. An exact assessment of the prevalence of CFC in the population cannot yet be determined as there is no data available from epidemiological studies or newborn screening [4]. Approximately 300 cases have been described in the literature.5 In addition, the reported prevalence in Japan is 1/810,000 people [2].
A 13-year-old boy was referred to our tertiary center, dermatology clinic for a sudden generalized eruption of multiple nevi throughout his skin. He reports it initially started several years prior to presentation and noted a sudden increase in the size, and number of his lesions in a short period. He denies any photosensitivity or history of prolonged sun exposure or sunburn. He denies any pruritus, erythema, edema, bleeding, ulceration or discharge of the lesions. He also denies any changes in color of the lesions. He denies any night sweats or unintentional changes in weight. He denies any personal or family history of melanoma or non-melanoma skin cancers. He has a 2-year history of palmoplantar hyperhidrosis but has not tried any treatments; thus far. His background medical history is notable for macrocephaly, polyneuropathy, cataracts and myopia, and is being followed by pediatric neurology and pediatric ophthalmology; respectively. He was initially seen by the pediatric neurology team 4 years prior to presentation due to a history of developmental delay and polyneuropathy. His gestational history was notable for polyhydramnios and macrocephaly on antenatal scan. He otherwise had a normal uneventful delivery at full-term. His developmental milestone history was notable for walking at 19-months-old and speaking at 4-years of age; all other milestones were within normal limits. He has a history of stool urgency that self-resolved by 9-years of age. He has no known allergies and is not on any medications. Family history was notable for consanguinity. His physical exam by the pediatric neurology team revealed macrocephaly with a head circumference of 56cm and hyperalgesia was noted physical exam but all other findings were within normal limits.
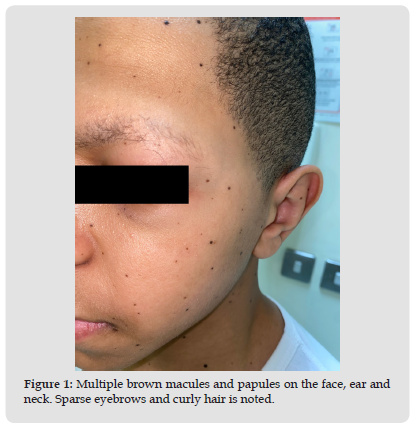
His laboratory work up revealed a vitamin-D insufficiency of 39 nmol/L but otherwise had a normal: complete blood count (CBC), comprehensive metabolic profile (CMP), urinalysis, erythrocyte sedimentation rate (ESR), antinuclear antibody (ANA), C3, C4, rheumatoid factor (RF), creatine kinase (CK), thyroid function test (TFT) and vitamin b12. Nerve conduction studies confirmed sensorimotor, predominately axonal polyneuropathy with no myopathy. Given his history and constellation of symptoms whole genome sequencing was conducted which confirmed a pathological variant, heterozygous, MAP2K2 mutation. Parental segregation analysis was negative thus, confirming sporadic MAP2K2 mutation.He was given vitamin-D supplementation, and managed conservatively with observation and continues to follow up with pediatric neurology for his polyneuropathy. He was eventually referred to dermatology for further evaluation and management of his nevi. At presentation in our dermatology clinic, he stands at 1.65m (<75th percentile) with a weight 44.5kg (50th percentile) and is vitally stable [7]. A full body skin exam was conducted that revealed, generalized xerosis, sparse eyebrows, curly hair, and multiple scattered <5mm, brown macules and papules throughout the scalp, face, ears, neck, trunk, and bilateral upper and lower extremities. (Figures 1-4) Approximately, more than 200 pigmented lesions were identified. Dermoscopy confirmed a regular pigment network without features of atypia namely, color variation, an irregular pigment or vascular network or white structureless areas that are typically seen in melanoma. There was no lymphadenopathy noted on exam. His findings are consistent with benign melanocytic nevi. Furthermore, a biopsy conducted at an outside hospital prior to presentation confirmed a benign melanocytic nevus. He was given emollients for his xerosis, topical antiperspirants for his palmoplantar hyperhidrosis and reassurance and education of his nevi and clinical course. He was instructed to observe for lesion asymmetry, border irregularity, changes in color or diameter, or evolutions of the lesions. These features are typically considered the ABCDE rule of early melanoma detection [8]. He was given follow up for regular once-yearly skin checks and to return sooner if warranted.
Figure 1 Multiple brown macules and papules on the face, ear and neck. Sparse eyebrows and curly hair is noted.
The RAS/MAPK pathway has been extensively studied for its role in oncogenesis. This pathway is composed of proteins that govern cell differentiation, proliferation, migration, and apoptosis. Mutations in this pathway can lead to dysregulation of these processes, resulting in uncontrolled cell proliferation and ultimately cancer [1-5,9]. CFC is caused by germline mutations along the Ras/MAPK cell signaling pathway. It can be further sub-classified based on the affected gene. BRAF mutations on chromosome 7q34 are seen in CFC type 1 and are the most common type reported in 75% of patients. Followed by, mutations in MAP2K1 (MEK1) on chromosome 15q22.31 (CFC type 3) and MAP2K2 (MEK2) on chromosome 19p13.3 (CFC type 4) both accounting for roughly 25% of patients. Followed lastly, by KRAS mutations on chromosome 12p12.1 (CFC type 2) in less than 2% of patients [5]. Patients with CFC may the show many overlapping clinical features with other RASopathies such as Noonan syndrome, Costello syndrome, Neurofibromatosis type 1 (NF1) and Legius syndrome, which may make diagnosis based on physical findings alone, difficult [6]. Thus, genetic testing is advised to confirm the diagnosis. Some of these entities may present with dysmorphic facial features in addition to abnormalities of multiple organ systems in association with skin manifestations of multiple pigmented lesions, namely, cafe au lait macules, lentigines and nevi [1-6].
Given, that somatic BRAF mutations have been reported to occur at a high frequency in patients with melanoma and other cancers; regular screening would be advised. Furthermore, it’s reported that the majority of benign melanocytic nevi and primary and metastatic melanoma have a specific; BRAF V600E mutations. This illustrates the importance of the RAS/MAPK pathway in the development of melanocytic neosplasms. [9] The literature suggests that the BRAF mutations in patients with RASopathies have less malignant potential than the V600E mutations [9]. Furthermore, mutations in MAP2K1 have been identified in ovarian cancers and mutations in both MAP2K1 and MAP2K2 have been identified in melanomas and lung cancers [9-13]. Finally, the literature indicates that KRAS is the most often mutated oncogene in humans, as it is frequently mutated in pancreatic, colorectal, cholangial, and lung cancers [14]. Patients with CFC have an uncertain prognosis, as current research is limited and there is no treatment available. Although the prognosis is uncertain, there have been no cases of melanoma reported in the literature in a patient with CFC to date [9]. In conclusion, consider genetic testing, a multidisciplinary approach, patient education and regular skin checks in a patient that presents with a constellation of findings in association with an eruption of multiple pigmented lesions on the skin.
The authors have no conflicts of interest to disclose.
This article has no funding sources.


