Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Kari Syrjänen1*, Markku Nissilä2, Mikko Kallela3 and Osmo Suovaniemi4
Received:February 08, 2023; Published:February 17, 2023
*Corresponding author: Kari Syrjänen, SMW Consultants Ltd, Kylliäisentie 9, FI-21620 Kuusisto, Finland
DOI: 10.26717/BJSTR.2023.48.007700
Background: Testimonials received from migraine patients stated that L-cysteine (Acetium®
Capsules) originally used for elimination of carcinogenic acetaldehyde in the stomach, prevented
also their headache attacks. These endorsements prompted the company (Biohit Oyj, Helsinki) to
formulate a hypothesis to explain these anti-migraine effects of Acetium® Capsules. It was reasoned
that elimination of acetaldehyde (a potent liberator of histamine from tissue mast cells) by L-cysteine
reduces the levels of histamine critically enough to arrest its availability to induce Nitric Oxide (NO),
which is considered as the final trigger of migraine attacks.
Randomized Controlled Trial (RCT): A double-blind, randomized placebo-controlled trial was
conducted in a multi-centre setting, where Acetium® capsules (100mg L-cysteine) were compared
with placebo during a 3-month trial period. The primary study endpoint was reduction in the
number of migraine days (NMD), with 4 cut-offs: ≥50%, ≥30%, ≥25% and ≥20% (28). Of the original
218 patients, 146 completed the study per protocol (PP). Using the most stringent cut-off (≥50%)
for NMD reduction, the global success rate in the Acetium® arm was 26.0% and in the placebo arm
25.4%; OR=1.027 (95%CI 0.589-1.79) (p=not significant).
Potential Efficacy in Special Subgroups: Given the known mechanism of Acetium® action
(elimination of acetaldehyde), subgroup analyses were focused on patients who reported potential
acetaldehyde-associated triggers of their migraine:
i) Alcohol,
ii) Cigarette smoking,
iii) Dietary
In these subgroup analyses, the highest RR=1.569 (95%CI 0.743-3.312) for Acetium® efficacy vs.
placebo was reached using the ≥20% cut-off for NMD reduction among subjects with a dietary
trigger (28). Albeit based on subgroup analyses, these results with Acetium® are worthy of attention
and prompt the investigators to continue further research.
Future Prospects: Although Acetium® was not more effective than placebo in reducing the NMD in all
migraine types, the results of the RCT are encouraging in that certain subgroups might benefit from
Acetium® intervention considerably more than migraine patients collectively. Highly interestingly,
clinical experience from one of the centres implicates that Acetium® use has been highly effective in
reducing the frequency of headache attack among subjects who suffer from hypnic headache (HH).
In the next step, plans have been made to proceed along two separate lines:
1) A registry-linkage study for migraine subgroups, and
2) A new RCT with cross-over design.
Keywords: Migraine; Attacks; Prevention; L-Cysteine; Acetaldehyde; Histamine Liberation; Acetaldehyde Elimination; Randomized Controlled Trial (RCT); Double-Blind; Placebo-Controlled; Number of Migraine Days (Nmd); Reduction of Nmd; Migraine Subgroups; Triggers; Alcohol; Smoking; Dietary; Hypnic Headache; Cross-Over Design; Parallel-Design
Abbreviations: CGRP: Calcitonin Gene-Related Peptide; AG: Atrophic Gastritis; PPI: Proton Pump Inhibitor; MTCA: 2-Methylthiazolidine-4-Carboxylic Acid; PKG: Phosphorylated Protein Kinase G; NO: Nitric Oxide; ECL: Enterochromaffin-Like; RCT: Randomized Controlled Trial; NMD: Number of Migraine Days; PP: Per Protocol; ICHD-3: International Classification of Headache Disorders
Prophylactic treatment is an essential part of management of
migraine patients, with two fold goals:
i) To reduce the frequency, severity, and duration of the
migraine attacks, and
ii) To increase the efficacy of abortive therapy [1-4].
The classic oral migraine prophylactics include β-adrenergic blockers, candesartan, amitriptyline, flunarizine, topiramate, sodium valproate and amitriptyline and botulinum toxin injections are used for chronic migraine [5]. The latest therapeutic innovations in migraine prophylaxis are based on monoclonal antibodies blocking the effect of calcitonin gene-related peptide (CGRP) [6-10]. While these treatments have been a success story [6], there are patients who do not respond and the cost of the monoclonal antibodies is substantial. New cost-effective oral treatments with no side-effects remain still high in the wish list of migraine patients.
A Potential New Tool for Migraine Intervention Emerged From Patient Testimonials
Completely unrelated to the progressive development of preventive and abortive drugs for migraine [1-10], a biotechnology company in Finland (Biohit Oyj) developed (in the early 2010’s) an innovative new tool for protection of gastric mucosa in patients with acid-free stomach, with the trade name Acetium® Capsule (L-cysteine) [11]. Acid-free stomach usually develops as a result of atrophic gastritis (AG) and/or long-term use of PPI (proton pump inhibitor) medication for dyspepsia, and consists a significant risk condition for gastric cancer (FG) [12]. The efficacy of Acetium® Capsule is based on the well-known chemical reaction whereby L-cysteine binds acetaldehyde (class I human carcinogen) [13] to form a chemically inactive and stable compound 2-methylthiazolidine-4-carboxylic acid (MTCA) [14]. Acetium® Capsule contains L-cysteine in a special slowrelease formulation, and the product has been demonstrated both safe and highly effective in eliminating carcinogenic acetaldehyde locally in the stomach [15-17]. When Acetium® capsules were administered to an increasing group of users in its original indication, the company started receiving testimonials from patients, who had noticed that their migraine attacks had either been aborted or reduced significantly. Several of those were interviewed, and some of the written statements are summarized in (Table 1). In addition, there were several others who were not willing to reiterate their experiences in written.
Table 1.Key clinical features of the migraine patients giving written testimonials* on the effects of Acetium® Capsules.
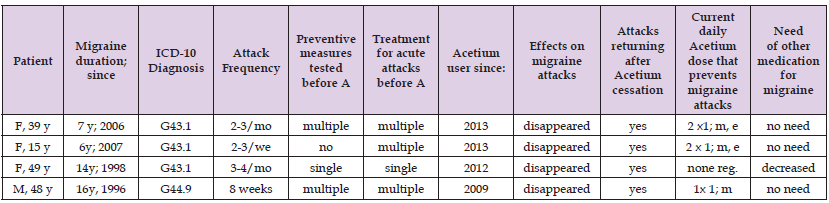
Note: *Testimonials received in 2013-2015 F, female; y, years; ICD, International Disease Classification; G43.1 migraine with aura (classical migraine); G44.009, cluster headache; mo, month; we, week; A, Acetium® capsules (100mg); m, morning; e, evening; reg., regularly
Case 1
A 36-year-old woman, whose migraine deteriorated in 2006 when the attacks started to be accompanied by aura as well as other symptoms e.g. weakness in the upper extremities. She was thoroughly examined, and the condition was diagnosed as hormonal migraine. She received a specific medication (Maxalt, Migard), but with some side effects and little effect on the attacks. Attacks continued appearing in 3-4 days a week, not infrequently severe enough to prevent daily work and other activities. Different physical therapies were of no help at all, and attacks continued with the same frequency. This led her to seek help from a wide variety of drugs, including Panadol and Panacod, with prolonged administration for protracted headache of over one week’s duration. Her situation gradually escalated to the stage when continuous use of analgesics was necessary to keep the headache at least in some control to enable daily work and other normal activities. She was advised to start the intake of Acetium® capsules on December 22, 2012, with 2 capsules in the morning and 2 in the evening. After a few months, she could reduce the dose to one capsule in the morning and one in the evening. To her major relief and surprise, all headache attacks have remained practically absent. During this period, she has had a few episodes, but all associated with a specific trigger (unrelated to her migraine). She does not need painkillers any longer, and if occasionally needed, they give a good relief. Interestingly, she once forgot taking Acetium® capsules for one week (due to non-availability), and the headache attacks returned immediately. She learned not to repeat that mistake, and at the time of this testimony (June 2016), she has remained attack-free for over 4 years.
Case 2
A 12-year-old girl, daughter of Case #1, who started suffering from severe headaches when 7 years old (in 2008). The attacks were characteristic to migraine, and in 2010, migraine with aura was diagnosed. A combination therapy with three painkillers was instituted, but with little help on the frequency of the attacks. The condition was aggravated in late 2012, with attack rate increasing up to 2-3 times a week. The migraine (possibly aggravated due to hormonal changes) diagnosis was confirmed by pediatric neurologist, who administered β-blockers for attack prophylaxis. The patient and her mother, however, decided to test the efficacy of Acetium® also for the daughter, with one capsule twice a day. Given the striking effect in her mother, the same effect was noticed also in the daughter, who has remained without a single migraine attack since the onset of Acetium® intake (April 2013). She feels herself completely healthy, fully capable of attending the school with no periods of absence ever since. Both the mother and her daughter have a feeling that with the use of Acetium® capsules, they have obtained a completely new life, with no concern of migraine on daily basis.
Case 3
A 41-year-old woman (from Norway), with a long-term history of severe migraine attacks who also has a diagnosed celiac disease and follows a strict gluten-free diet. Otherwise generally healthy, with no regular medication, except occasional anti-depressants. She started the daily use of Acetium® capsules as soon as they entered into the market, and after a few weeks, her migraine attacks disappeared. She has been attack-free now for years, and continues taking one Acetium® capsule every day for maintenance prophylaxis.
Case 4
A 48-year-old male, with cluster headache (Horton’s syndrome). He provided a very detailed description of his headaches, which fulfills the criteria of a classical cluster headache, diagnosed by his physician some 16 years ago. The attacks start at distinct time of the day (at 1-2 o’clock at night in his case), are unilateral and have a maximum duration of two hours. Some 6-7 years ago, the condition was aggravated and the attack rate increased, extending to other seasonal periods that during the first few years. The cluster periods lasted up to 8 weeks, having a definite negative impact on the quality of his daily life. He also listed the most important triggers of the clusters, including stress, associated with short sleeping hours and use of even small amounts of alcohol. During the years, he has tested several different modalities for cluster prophylaxis. The best combination, with some reduction in cluster frequency but not total disappearance, proved to be magnesium food supplement, melatonin (5mg), abundant drinking of water before going to sleep, refrain from alcohol intake, and highly regular sleeping. As said, the cluster frequency diminished, but the attacks did not disappear.
He started taking Acetium® capsules in December 2009, following a very intense cluster. Since then, he has continued taking one capsule in the morning. Occasionally, while feeling prodromal symptoms, he has raised the dosage to 2-4 capsules per day. Some six months since initiation of Acetium®, he was able to abandon his daily routines (described above), including the use of magnesium supplement, melatonin drinking abundant water before going to sleep. Interestingly, also the previous triggers of his clusters (stress, sleeplessness, alcohol), have not evoked new attacks since the onset of Acetium® prophylaxis. According to his written testimonial (July 2013), this patient has experienced not a single attack of cluster headache since December 2009, when he started the daily intake of Acetium® capsules, one capsule per day.
The Hypothesis Formulated to Explain the Anti-Migraine Effects of Slow-Release L-Cysteine
Given the known mechanism of action of slow-release L-cysteine,
a solid hypothesis was formulated to explain the reported antimigraine
effects by the patients. This hypothesis consists of several
elements, all based on solid experimental and/or clinical evidence.
These include both
i) The known pathways involved in the provocation of a
characteristic migraine attack, as well as
ii) Established and postulated mechanisms, how L-cysteine
administration could interfere with this sequence of events, leading
to vasodilatation and acute migraine attack.
Nitric Oxide (No) is the Final Trigger of Migraine Attack
NO binds to guanylyl cyclase in vascular smooth muscle cells, leading to synthesis of cyclic GMP, which in turn forms phosphorylated protein kinase G (PKG). PKG phosphorylates Ca2+ channels, slowing the influx of Ca into the cell, which leads to smooth muscle relaxation and vasodilation, resulting in characteristic migraine attack [18].
Histamine Induces Nitric Oxide (No) Synthase
There is experimental and clinical evidence implicating that histamine induces the enzyme Nitric Oxide (NO) Synthase, making NO available locally on the vasculature to act as a vasodilator. Histamine is known to activate cerebral endothelial H1-receptors, leading to formation of NO [18-21].
Histamine is Synthesized in Tissue Mast Cells and Basophils
Histamine is synthesized in tissue mast cells and basophils by histidine decarboxylase enzyme converting histidine to histamine. Another important source of histamine are enterochromaffin-like (ECL) cells that are abundant in gastric (corpus) mucosa. Histaminic cephalalgia is the old name for cluster headaches, implicating that histamine has been linked with the development of vascular headaches since their description [1-5]. Mast cells are ubiquitous, and their activation (e.g. in the meninges) by migraine triggers is believed to contribute to genesis of migraine headaches [22].
Histamine is Liberated from Tissue Mast Cells by Acetaldehyde
Tissue mast cells play a crucial role in hypersensitivity, allergic, and inflammatory reactions by secreting chemical mediators, e.g. histamine, proteases, and cytokines as a response to various immunologic and non-immunologic stimuli. One of the potent liberators of histamine from tissue mast cells is acetaldehyde [13], both in the human and in experimental animals [23,24]. This could neatly explain several hypersensitivity-like reactions associated eg. with alcohol intake and smoking, both being abundant sources of acetaldehyde [13-18].
Acetaldehyde in the Stomach and Saliva is Inactivated by L-Cysteine
The inherent property of L-cysteine to eliminate free acetaldehyde by reacting covalently with it to form a stable MTCA [14], was patented by Biohit Oyj in 1992, leading to the design of their Acetium®-line of products (capsule and lozenge) [11]. Both the Acetium® Capsule and the subsequently introduced Acetium® Lozenge (5 mg L-cysteine) effectively eliminate acetaldehyde derived from alcohol and/or cigarette smoke, both in the stomach and in the saliva [15-17,25,26].
The Efficacy of l-Cysteine in Migraine Prophylaxis?
It is highly unlikely that the dramatic disappearance of migraine
attacks and cluster headaches soon after regular intake of Acetium®
Capsules reported in the case histories (Table 1) would be simply by
change. Instead, it sounds more feasible that the mechanism must
be based on the capacity of L-cysteine to interfere with the above
described sequence of events:
i) Elimination of acetaldehyde in the stomach could
ii) Arrest histamine liberation from the tissue mast cells and
ECL cells in the stomach, thus
iii) Blocking its multitude of functions, of which
iv) Vasodilatation is critically involved in inducing the migraine
attack [18-22].
In the nervous system, histamine acts mainly on H1- and H3- receptors [27]. While H1-receptors mediate inflammation, H3- receptors are much more sensitive to histamine and serve as negative feedback to inhibit further excessive release of histamine by C-fibres (i.e., afferent fibres of the somatic sensory system) [28]. It is tempting to speculate, whether reduction of total histamine burden by eliminating acetaldehyde would be equivalent to so called “modulation of histamine” that has been successfully employed in the treatment of migraine and cluster headaches for several years [18]. Indeed, for migraine, the H3 -receptor-mediated negative feedback loop has been exploited by intermittent very low doses of sc. histamine. In theory, low histamine concentrations in the body would lead to preferential stimulation of the more sensitive H3 -receptors, while leaving the H1-receptors relatively untouched, thus activating the negative feedback loop and reducing the potential of the C-fibres to become activated [18].
To test the new hypothesis, a formal randomized controlled trial (RCT) was designed and conducted in a multi-centre setting [29]. The study design followed the guidelines published by the International Headache Society Clinical Trials Subcommittee [30]. In brief, this double-blind, randomized placebo-controlled trial compared Acetium® Capsules (100mg L-cysteine, twice a day) with placebo in prevention of migraine attacks during a 3-month trial period. Between 2015-2018, a cohort of 218 consented adult patients suffering from clinically confirmed migraine (ICHD-3 beta) with or without aura, were enrolled by 6 clinics in Finland and 2 clinics in Estonia. The eligible patients should:
i) Have the attack frequency of 2–8 days per month,
ii) Have had migraine for at least 1 year,
iii) Have the onset of their migraine before 50 years of age,
iv) Be between 18 and 65 years of age, and
v) Have a minimum or no co-morbidity [29].
The headache diary was used to monitor the efficacy of the test preparations at 1-month intervals. The primary study endpoint was reduction of the number of migraine days (NMD) per month [29].
Of the originally enrolled 218 patients, 146 completed the study per protocol (PP), and thus eligible for the final analyses [28]. In the global analysis, no statistical difference between the two study arms was detected using ≥50%, ≥30%, ≥25% and ≥20% cut-offs in reduction of NMD. Using the most stringent cut-off (≥50%), the success rate in the Acetium® arm was 26.0% and in the placebo arm 25.4%; OR=1.027 (95%CI 0.589-1.79)(p=not significant).
Migraine Subgroups of Special Interest
Subgroup analyses for NMD reduction were completed for those
patients who reported identifiable acetaldehyde-associated triggers
of their headache attacks:
i) Alcohol,
ii) Cigarette smoking,
iii) Dietary [28].
Given the mechanism of Acetium® action (i.e., elimination of alcohol and cigarette smoke-derived acetaldehyde)(14-17,25,26), it was of interest to find out that Acetium® was >10% more effective than placebo in inducing NMD reduction by 20% cut-off; 61.8% vs. 50.0% (RR=1.235; 95%CI 0.805-1.895)(p=0.328), among those patients who reported alcohol intake as a trigger of their attacks. Results were even more remarkable among the rare patients (n=5) with clear cigarette smoking as a trigger, of whom 66.7% achieved ≥50% reduction in NMD with Acetium® as compared with 0% for those using placebo, respectively. These differences are not statistically significant because of the fact that the numbers of subjects in these subgroups are far too small to reach statistical significance [28]. There are good chances, however, that with an adequately powered cohort, these results in the subgroup analysis should reach statistical significance. For the above listed differences to become significant, 278 subjects in each study arm would be needed in the alcohol-trigger subgroup (61.8% vs. 50%)(28), whereas only 7 subjects per study arm would make the difference 66.7% vs. 0.0% significant in the smoking-trigger subgroup. In our RTC, this subgroup of smoking-triggers included only 3 and 2 subjects in the Acetium® and placebo arms, respectively [28].
Hypnic Headache (HH)
Patients with hypnic headache (HH) were not included as a
special subgroup in our RTC, but this group of subjects has aroused
interest in this respect afterwards as explained later. HH is a rare
headache syndrome that occurs exclusively during sleep, usually at
the same time at night. It also has been named before the clockwise
headache or alarm clock headache [30]. According to the international
classification of headache disorders (ICHD-3) [31], HH is considered
as a primary headache defined as
1) Headaches that develop only during sleep and cause
awakening,
2) Occur greater than or equal to 10 days per month for greater
than or equal to 3 months,
3) Last greater than or equal to 15 minutes for up to 4 hours
after awakening, and
4) Have no associated cranial autonomic symptoms or
restlessness.
HH is characterized by attacks of dull headaches that occur at any age (predominantly after the age of 50 years), at least 15 times per month, and come only during sleep with no associated autonomic symptoms. The attacks wake the patient during sleep and usually last more than 15 minutes after waking [30,31]. HH is a chronic disorder that can last for years, with no remission of headache. Patients with migraines and hypertension are more likely to develop HH compared to others [30,32,33]. Nearly all patients with HH had some type of motor activity, such as getting out of bed and eating, drinking, showering, or reading when awakened by the headache attack. Still, they did not typically have the restlessness that is seen with cluster headache and trigeminal autonomic cephalalgias [30]. The etiology of HH is unknown. HH might be associated with issues in the parts of the brain involved in pain management, melatonin production, or REM sleep [32,33]. HH is encountered in about 0.07% of all patients attending the doctor due to headache complaints. However, among geriatric populations, this ratio is higher, up to 1.4% [30]. Interestingly, in more recent studies, HH seems to be more common than previously expected, with estimates ranging from 0.3% to 0.6% e.g. in France [34].
HH has been included in this discussion because of the simple reason that tentative (unpublished) clinical evidence based on individual HH patients (between 70 to 100) suggests that Acetium® administration has been effective in preventing their nocturnal headache attacks [35]. According to this experience from one of the clinical centres participated in the RCT [28], neurologists started administering Acetium® Capsules to migraine patients in parallel with the ongoing RCT. It soon appeared that a favourable response was reported by an increasing number of subjects whose headache was classified as HH. During the past years, the number of these HH patients has steadily increased, but the exact number is not available without systematic registry search. Prompted by this favourable feedback, the clinicians are planning to apply the formal permission to conduct a registry-linkage study where all subjects with diagnosed HH (and the above defined subgroups) in the clinic will be identified, and their clinical records analysed with regard to the Acetium® administration and the objective response to it [35].
Future Prospects with Acetium® and Migraine
As discussed above, the results in subgroups of the first RCT were encouraging [28], although in the global analysis of the two study arms, Acetium® capsules were not more effective than placebo in reducing NMD in migraine patients [28]. This result was not exactly what was expected on the bases of the positive testimonials from the migraine patients (Table 1). To the investigators, these data suggest that Acetium® capsule is not effective in prevention of all types of migraine. Given the complexity of migraine pathophysiology, this conclusion sounds even predictable, because it is unlikely that any single drug will ever be a perfect remedy for all types of migraine [1- 4]. Keeping in mind the known mechanism of Acetium® action (i.e., local elimination of acetaldehyde) [15-17,25,26], it is tempting to pay attention to migraine patients who report a potential acetaldehydeassociated trigger of their migraine attacks [28]. When this was done by analysing the subgroups of the patients in the RCT [28], some intriguing observations were made as cited above. In these subgroup analyses, the highest RR=1.569 (95%CI 0.743-3.312) for Acetium® efficacy vs. placebo was reached using the ≥20% cut-off for NMD reduction among subjects with a dietary trigger [28]. These results with Acetium® clearly prompt the investigators to continue additional research in patients with a certain migraine trigger.
At present, plans have been made to proceed along two separate
lines:
1) A registry-linkage study for migraine subgroups, including
HH and
2) A new RCT with cross-over design. Mention was already
made above concerning the positive response with Acetium® reported
by the patients with HH [35].
To get clinically validated data for formal statistical analysis, a registry-linkage study is needed, focused not only to HH but also including the special migraine subgroups discussed above (i.e., those with alcohol-, smoking- or dietary trigger). A formal permission is needed from the regulatory authorities to perform a registry-linkage study, and the application is currently pending. As to the RCTs with any prophylactic migraine trials, discussion continues as to the advantages and disadvantages of the two main study designs (parallel design and cross-over design), the fluctuating course of migraine posing similar challenges in both designs, unfortunately [2,29]. After careful consideration, our first RCT was conducted using the parallel design [28]. Despite the prior power calculations, the statistical power of our RCT finally proved to be inadequate particularly in the subgroup analysis [28], where the size of the strata should be larger. A good example is the RR-calculation for the dietary triggers cited above, with RR=1.569 (95%CI 0.743-3.312): an adequate statistical power would be reached with 86 subjects in both study arms, instead of the actual 16 and 17 that were available in the original study [28].
Given that the enrolment of the subjects in the first trial took much longer than expected, a significant increase of the cohort size in the next RCT is not an attractive option, however. One solution could be to use cross-over design instead of parallel design, because the former is up to eight times more powerful than the parallel-group design in prophylactic migraine trials [36]. Higher study power translates into a lower sample size, which has the advantage of exposing fewer participants to investigative drugs. This “power advantage” of the cross-over design is countered by some shortcomings, including the possible carryover effect [37]. In the case of Acetium® Capsules, however, this is not an issue because the active substance (L-cysteine) is acting locally in the stomach and not absorbed into the circulation [11]. Yet another important aspect favouring the cross-over design is associated with the complexity of migraine syndrome itself. Only a cross-over design can avoid the potential bias that different-type migraines (with different response to any medication) are allocated by chance to the two parallel groups, thus precluding an unbiased estimation of the drug efficacy [2,29,36]. All these advantages favour the view that the next RCT with Acetium® Capsule in migraine prophylaxis should be conducted using a cross-over design.
At present, a lot of promises are being placed on Acetium® as a potential new therapeutic in migraine prophylaxis. One formal RCT (using parallel-design) was already conducted for migraine patients [28] and another one for the subjects with cluster headache [38]. A new RCT is planned using a cross-over design to increase the statistical power. As suggested by the data from the first RCT as well as the newly accumulated clinical evidence on the efficacy of Acetium® in HH, future studies will be clearly focused on HH and migraine patients in whom an external trigger can be identified that is related to acetaldehyde metabolism [39]. Eliminating acetaldehyde is the primary mode of action of Acetium® capsules, and this offers a feasible explanation why the patients in these subgroups seem to benefit from Acetium® Capsules substantially more often than other migraine patients. More data are needed to uncover the potential mechanisms that would explain the efficacy of Acetium® prophylaxis in subjects with HH. A planned comprehensive registry-linkage study hopefully gives further elucidation to this. Given that L-cysteine is a natural (semi-essential) amino acid, converted to an inert substance (MTCA) upon reaction with acetaldehyde in the alimentary tract, Acetium® capsule would comprise an ideal means to conduct migraine prophylaxis for years without concern about the side effects inherent to many of the currently used preventive medicines [1-10,28]. If additional studies provide confirmatory evidence on the efficacy, the concept of using Acetium® Capsules in prophylactic treatment of migraines would represent a major step forward in a better clinical control of these frequently intractable symptoms.


