Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Nan Shen1#, Hongyu Miao1#, Yudong Fu2, Jielu Pan1, Ruiqing Wang1, Yucheng Fang1, Haiyan Zhang1, Xiao Yu1* and Lianjun Xing1*
Received: February 06, 2023; Published: February 15, 2023
*Corresponding author: Xiao Yu and Lianjun Xing, Department II of Digestive Diseases, Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China
DOI: 10.26717/BJSTR.2023.48.007690
A 67-year-old man was diagnosed with gastric signet ring cell carcinoma with hepatocellular carcinoma. Hepatocellular carcinoma with a major portal vein tumor thrombus and a right atrium tumor thrombus is an unresectable advanced tumor with limited treatment options. The combination of atezolizumab plus bevacizumab was initiated, and the patient’s survival was significantly prolonged after 20 courses of treatment. The cases of gastric cancer complicated with primary liver cancer are rarely reported at home and abroad, the treatment options are limited, and the survival time is short. We report here a case of atezolizumab plus bevacizumab in the treatment of gastric signet ring cell carcinoma with hepatocellular carcinoma.
Keywords: Gastric Signet Ring Cell Carcinoma; Hepatocellular Carcinoma; Atezolizumab Plus Bevacizumab; Case Report
Abbreviations: MPC: Multiple Primary Cancer; HCC: Hepatocellular Carcinoma; GSRCC: Gastric Signet Ring Cell Carcinoma; PVTT: Portal Vein Tumor Thrombus; PFS: Progression Free Survival
Multiple primary cancer (MPC) refers to the occurrence of two or more mutually independent primary malignancies in a single or multiple organs of the same individual, either simultaneously or sequentially [1]. The incidence of multiple primary cancer has been reported in the literature to be 2.7%-10.6% [2]. Studies have shown that patients with malignant tumors have a 6-fold or even 12-fold higher chance of developing a second cancer than normal, with MPC in the digestive system being the most common [3]. It is rare to find a primary hepatocellular carcinoma (HCC) in combination with a gastric signet ring cell carcinoma (GSRCC) at a different time. HCC with major portal vein tumor thrombus (PVTT) and right atrial tumor thrombus is an extremely advanced tumor with limited treatment options [4]. Especially after invasion of the main or contralateral portal trunk, HCC with PVTT has a poor prognosis due to potential intrahepatic metastases through the portal vein and deterioration of liver function due to reduced portal blood flow [5]. Current guidelines recommend systemic therapy for such advanced tumors, and atezolizumab plus bevacizumab is the first-line agent for unresectable HCC [6]. The results of the IMBrave150 global multicenter phase III study [7] showed that the median survival time and progression free survival (PFS) in the atezolizumab plus bevacizumab group were higher than those in the sorafenib group [8]. PFS were significantly prolonged, with a 34% reduction in the risk of death and a 35% reduction in the risk of disease progression, compared with the sorafenib group. For the Chinese subgroup, patients in the combination therapy group also showed significant clinical benefit, with a 47% reduction in the risk of death and a 40% reduction in the risk of disease progression compared with sorafenib. Recently, a patient with primary HCC combined with GSRCC was admitted and is reported below.
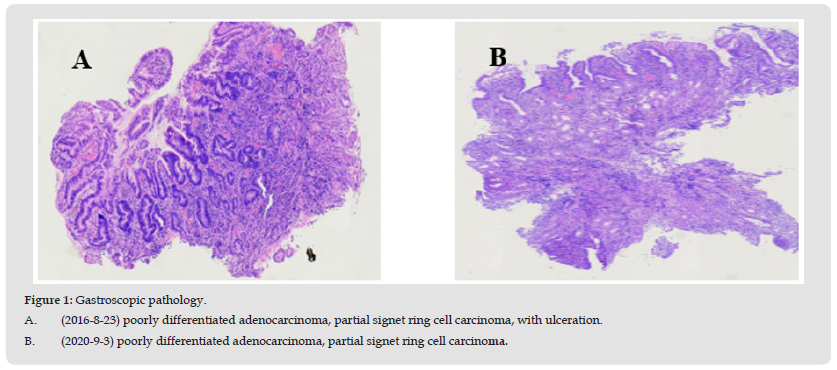
The patient is a 67-year-old male. He was admitted to hospital on August 23, 2016 due to «recurrent epigastric vague pain for half a month». He had a previous history of hepatitis B and was not under standard treatment. Physical examination: no yellow staining of skin and sclera, hepatic palms and spider nevus were visible, abdomen was flat, deep pressure discomfort in right upper abdomen. Laboratory examination: AFP 16.52ng/ml, CEA6.69ng/ml, ALT43IU/L, AST65IU/L. Gastroscopy: A 2.0*0.5cm strip-like elevation was seen in the gastric horn, with surface congestion and hard texture; a circular patchy erosion was seen on the side of the gastric body near the gastric sinus, about 1.5*1.5cm, with punctate bleeding on the surface and soft texture. Pathology: gastric horn: chronic nonatrophic gastritis with activity; gastric body: poorly differentiated adenocarcinoma, partial signet ring cell carcinoma, with ulceration (Figure 1A). Immunohistochemistry: Cam5.2(+), C-erBb-2(++), Ki- 67(60%), AE1/AE3(+). Gastroscopy was repeated on September 3, 2020: esophageal varices, chronic atrophic gastritis with augmented erosion (gastric sinus and gastric body predominant), gastric horn ulcer stage H1. Pathology: gastric horn: poorly differentiated adenocarcinoma, partial signet ring cell carcinoma; gastric body: chronic inflammation of superficial mucosa (Figure 1B). No surgical treatment was performed.
Figure 1 Gastroscopic pathology. A. (2016-8-23) poorly differentiated adenocarcinoma, partial signet ring cell carcinoma, with ulceration. B. (2020-9-3) poorly differentiated adenocarcinoma, partial signet ring cell carcinoma.
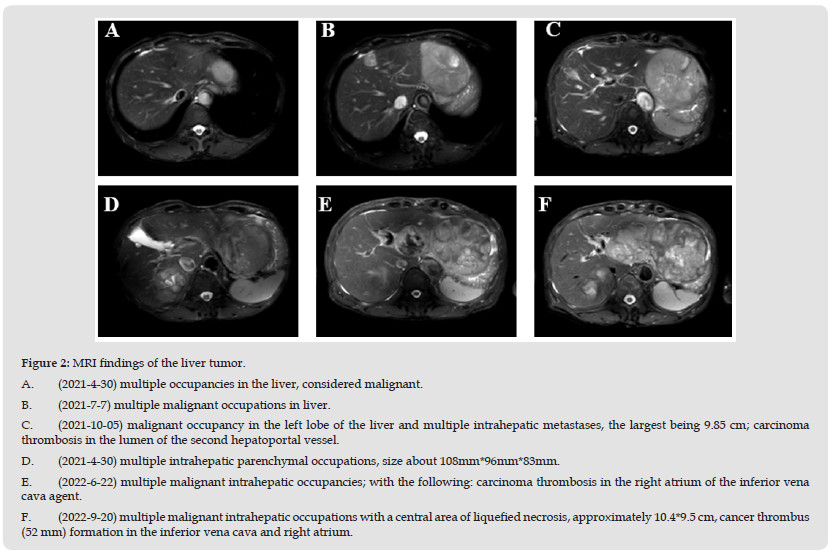
Posterior epigastric vague pain recurring from time to time, investigated epigastric enhanced MRI on April 30, 2021: multiple occupancies in the liver, considered malignant; multiple cysts in the liver; cirrhosis (Figure 2A). AFP 8.25ng/ml. Repeat upper abdominal enhancement MRI on July 7, 2021: multiple malignant occupations in liver, enlarged from April 29, 2021, cysts in liver and both kidneys, cirrhosis (Figure 2B). AFP13.94ng/ml. Combined with the patient’s history of hepatitis B, AFP and imaging examination, multiple hepatic occupying was considered to be caused by primary liver cancer, and the diagnosis was as follows:
1. Primary liver cancer (HCC).
2. Primary gastric poorly differentiated adenocarcinoma (GSRCC) is a synchronous primary carcinoma.
Atezolizumab plus bevacizumab was used on July 12, 2021. Considering that the patient had taken warfarin for long-term anticoagulation after previous lower limb venous thrombosis filter implantation, Atezolizumab full dose of 1200mg combined with bevacizumab reduced dose of 400mg was selected for treatment. There were no adverse reactions such as fever, pain and rash after the first dose. Abdominal B-ultrasonography 3 weeks after the first administration showed no significant increase in the size of the major tumor or PVTT. Serum AFP changed from 13.94 ng/mL to 21.79 ng/mL and 18.65ng/mL before treatment at 3 and 5 weeks after Atezolizumab combined with bevacizumab, respectively. The MRI of the upper abdomen was repeated on October 5, 2021 after 3 months of drug administration: cirrhosis with malignant occupancy in the left lobe of the liver and multiple intrahepatic metastases, the largest being 9.85 cm; carcinoma thrombosis in the lumen of the second hepatoportal vessel (Figure 2C). On January 20, 2022, the MRI of the upper abdomen was reviewed: multiple intrahepatic parenchymal occupations, size about 108mm*96mm*83mm, small amount of ascites, small cysts in the liver and right kidney (Figure 2D). On June 22, 2022, the MRI of the upper abdomen was reviewed: multiple malignant intrahepatic occupancies; with the following: carcinoma thrombosis in the right atrium of the inferior vena cava agent (Figure 2E). AFP 224.2 ng/ml. On September 20, 2022, the MRI of the upper abdomen was reviewed: multiple malignant intrahepatic occupations with a central area of liquefied necrosis, approximately 10.4*9.5 cm, cancer thrombus (52 mm) formation in the inferior vena cava and right atrium (Figure 2F). AFP531.9ng/ml.1st-20th doses of atezolizumab plus bevacizumab (atezolizumab 1200mg, D1, Q3W+ bevacizumab 400mg, D1, Q3W) were administered from July 12, 2021 to January 14, 2023. Regularly assessed for stability and maintenance therapy. The patient died on January 15, 2023 due to dislodged cancer thrombus.
Figure 2 MRI findings of the liver tumor. A. (2021-4-30) multiple occupancies in the liver, considered malignant. B. (2021-7-7) multiple malignant occupations in liver. C. (2021-10-05) malignant occupancy in the left lobe of the liver and multiple intrahepatic metastases, the largest being 9.85 cm; carcinoma thrombosis in the lumen of the second hepatoportal vessel. D. (2021-4-30) multiple intrahepatic parenchymal occupations, size about 108mm*96mm*83mm. E. (2022-6-22) multiple malignant intrahepatic occupancies; with the following: carcinoma thrombosis in the right atrium of the inferior vena cava agent. F. (2022-9-20) multiple malignant intrahepatic occupations with a central area of liquefied necrosis, approximately 10.4*9.5 cm, cancer thrombus (52 mm) formation in the inferior vena cava and right atrium.
Liver cancer is one of the malignant tumors with the highest incidence in the world, and more than half of the new cases of liver cancer reported every year occur in China. Gastric cancer is also a common malignant tumor, but the double cancer of primary liver cancer combined with gastric cancer is rarely reported in the literature at home and abroad. Shah et al. [8] reported 466 cases of multiple primary cancers complicated with primary liver cancer (1992-2011) from the American Cancer Epidemiology Database, among which only 20 cases were complicated with gastric cancer. Li Zhe et al. [9] from Eastern Hepatobiliary Surgery Hospital reported 14 cases of primary liver cancer complicated with gastric cancer, the largest number of cases reported in China, among which only 1 case was complicated with signet ring cell carcinoma. The pathogenesis of multi-primary cancer is relatively complex, and the etiology is not clear at present, which may be related to genetic factors, immune factors, iatrogenic factors (chemotherapy and ionizing radiation), environment and lifestyle [10]. In patients with a prior history of cancer, it may be difficult to establish the diagnosis of additional primary tumors. Heterotemporal gastric signet ring cell carcinoma combined with primary liver cancer is rare at home and abroad, and it is often diagnosed as gastric cancer liver metastasis or liver cancer invading the gastric wall, which is easy to cause misdiagnosis [11]. Intensive follow-up after treatment of the first primary cancer is essential for early diagnosis, thus avoiding late diagnosis of the second cancer.
Only early detection allows for early diagnosis and treatment as well as improved treatment outcomes and prognosis. When two active malignancies are diagnosed in a single patient, the challenge is to find an anticancer treatment strategy that covers both cancer types without increasing toxicity or associated pharmacological interactions, and without negatively affecting the overall outcome. We report a case of GSRCC complicated with HCC complicated with PVTT and right atrial cancer embolus. In combination with atezolizumab plus bevacizumab, serum AFP levels increased slowly, tumor progression was significantly controlled, and survival time was prolonged without adverse reactions. Antithrombotic therapy continues, as the efficacy of antithrombotic therapy for HCC with PVTT has been reported in the literature [12]. The clinical course of this case was surprising because of the strong potential of atezolizumab plus bevacizumab in the treatment of HCC with advanced PVTT. In tumors with PVTT, the Japanese cancer research group found that it could be fatal within 2 weeks as a clinical emergency, because it took only 11.5 days for PVTT to progress from the ipsilateral branch of the first portal vein to the main portal vein [13]. It has been reported that the median survival time of sorafenib in PVTT HCC patients is only 3-4 months [14]. The survival time of the case we report is 18 months. In the IMbrave150 trial, the objective rate and disease control rate of atezolizumab plus bevacizumab were 33.2% and 72.3%, respectively [7].
IMbrave150 includes high-risk patients, defined as PVTT patients, tumors that comprise more than 50% of the liver, and patients with bile duct invagination. Recently, it was reported that the median survival time of high-risk patients treated with Atezolizumab combined with bevacizumab was only 7.6 months, while the survival time of nonhigh- risk patients was 22.8 months [7]. This case demonstrates the powerful potential of atezolizumab plus bevacizumab to induce a large tumor response. The novelty of this case should be recognized and its potential as a PVTT therapy for liver cancer needs more attention. The introduction of immune checkpoint inhibitors resulted in changes typical of systemic therapy for HCC. Further analysis of outcomes in patients with HCC and severe vascular invasion is warranted. With the development of analyses for predicting the effect of treatment prior to the introduction of chemotherapy, more complex tailored treatments may emerge in the future, including the selection of patient groups that would benefit from systemic treatment.
In conclusion, although the patient was unable to undergo surgery or systemic treatment for GSRCC due to his own reasons in this case, the patient was found with HCC in a timely manner due to close follow-up and received appropriate treatment actively, thus prolongating the patient’s survival time. As the cases are relatively rare, timely diagnosis and active treatment have brought great benefits to the patients.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
The patient provided his written informed consent to participate in this study.
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
NS and HM contributed to the conception, design and manuscript; NS, YF, HM, JP and RW were involved directly or indirectly in the care of the patient; HZ, XY and LX participated in the collection of case data. All authors contributed to the article and approved the submitted version.
This research was funded by the Science and Technology Innovation Action Plan of Shanghai Science and Technology Commission (No. 20y21902400) , Longhua Medical Scholar (No. PY2022017), the Training Plan of Famous Traditional Chinese Medicine Doctors in Pudong New Area of Shanghai (No. PWRzm2020-03), the Shanghai University of Chinese Medicine reserve outstanding TCM talents (No. 2020012) and Shanghai Municipal Health Commission and Shanghai Medical and Health Development Foundation, General Practice Project of New Star Young Medical Talents in Medical Sciences (No. 2020087), Shanghai 2022 «Science and Technology Innovation Initiative» medical innovation research project (No. 22Y11921500), Construction of demonstration center for traditional Chinese medicine non-drug therapy (2021-2023) (No. ZY (2021-2023) - 0204-08).
Not applicable.


