Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Gedion Goshu1, Bikila Abebe1* and Firomsa Dinka2
Received: February 02, 2023; Published: February 14, 2023
*Corresponding author: Bikila Abebe, Jimma University, College of Agriculture and Veterinary Medicine Po Box; 378, Jimma, Oromia, Ethiopia
DOI: 10.26717/BJSTR.2023.48.007689
Calf mortality is perennial problems for dairy producers worldwide, which impair appropriate heifer replacement. The common diseases that cause death in young calves were diarrhea and pneumonia, even though other diseases like novel ill, arthritis, bloat, septicemia, arthropod parasites, and nutritional diseases were also incriminated. A Longitudinal prospective observational study was conducted between November 2016 to April 2017 in urban and peri-urban small holder dairy farms of Bishoftu town on 158 live born calves selected from 60 dairy farms to identify the risk factors associated with calf mortality in cross-breed small holders in an intensive and semi-intensive dairy production systems. Questionnaire tool was used to collect information on the risk factors of calf mortality. Calf diarrhea was the leading cause of calf mortality with case-specific mortality of 3.1% among the causes of death recorded. The total cumulative incidence of mortality found in this study was 8.2 %. The association of 8 potential risk factors with dairy calf mortality was investigated. Of these factors, among others, delivery condition (OR=5.9, P=0.018), amount of colostrum (2L) (OR =0.17, P = 0.039), Age of calf (OR=6.5, P=0.046), and farm size≥8 (OR = 12.9, P = 0.007), were the risk factors found to be significantly associated with the death of calves. The present study suggested that the existence of high mortality in small dairy farms might be due to poor farm and calf management. Hence, special emphasis should be given to the amount of colostrum feeding; proper management as the farm size increases and especial care management of the first week age of calves were found important. Moreover, further investigation is suggested to identify the specific causative agents incriminating for calf mortality in dairy farms of the study area.
Keywords: Bishoftu; Calves; Mortality; Risk Factors; Smallholder Dairy Farms
Livestock production consists one of the principal means of achieving improved living standards in many regions of the developing world. In sub-Saharan African countries, livestock plays a crucial role both for the national economics and the livelihood of rural communities (ILCA [1]). However, the development of this sector is very gradual in countries of sub-Saharan Africa. Calf morbidity and mortality are perennial problems for dairy producers worldwide (Heinrichs and Radostits, et al. [2]), especially the tropics is not an ideal location for calf rearing as the high temperature and humidity introduce many potential disease problems to milk-fed calves (Moran, et al. [3]) which impair appropriate heifer replacement. Heifer replacement markedly influences the ability of dairy man to increase production by allowing him to practice selective culling of low productive cows (Waltner–Toews, et al. [3,4]). The two primary diseases are diarrhea and pneumonia (Virtala, et al. [5]), and are the commonest disease in young calves (Sivula, et al. [6]) and pre-weaned calves (Heinrichs and Radostits, [2]), respectively, even though other diseases like navel ill (Kasari, et al. [7]), arthritis, bloat, septicemia, arthropod parasites, and nutritional diseases are also reported (Heinrichs and Radostits, et al. [2]). Given the considerable potential for smallholder income and employment generation from high-value dairy products, development of the dairy sector in Ethiopia can contribute significantly to poverty alleviation and nutrition in the country. However, The success of any breeding program as well as the future of smallholder dairy farms depends upon the rate of survival of the calf crop produced and accordingly calf mortality and morbidity are of great concern to dairyman, because most of the dairy farms are confronted with acute problems of calf mortality (Wudu, et al. [8,9]).
Smallholder dairy farmers experience high calf mortality which can go up to 50% (Moran, et al. [3]). Neonatal period (0-28 days) is of critical importance because most of the deaths occur within the first 2 weeks of life (Wells, et al. [10]). During the first few weeks of life, when calves are typically fed whole milk or milk replacers, it is also the time when calves experience episodes of diarrhea, often caused by infectious organisms such as Rotavirus, , Escherichia coli, Cryptosporidium parvum or Salmonella spp. (Ambrosim, et al. [11,12]). Calf death inflicts enormous economic losses on cattle farmers all over the world (Lundborg, et al. [13]). Economic losses are not only associated with death but also with loss of genetic material, intervention cost, loss of performance, and productivity later in life (Wells, et al. [10,13]). Same studies conducted on calf mortality 0-1 years in Ethiopia show mortalities that ranged from 3.6 to 30.7% (Wudu et al. [8,14]). Thus, identification of factors which can alter a calf’s risk of death is an important prerequisite for avoiding excessive calf mortality (Debnath et al. [15]). Calf diseases that cause mortality are the result of complex interactions of the management practices, environment, infectious agents, and the calf itself. Implementation of improved calf management practices is greatly suggested to reduce the high level of calf disease problems (Wudu, et al. [8]). Although there were some research works carried out in the study area on the problem of calf morbidity and mortality which mostly focus on large and research dairy farms. Since the area is highly increasing in small holder dairy farms and calf mortality is a series problem, the present study focused mainly on small holder dairy farms with the objectives of;
To evaluate neonatal calf mortality rate in small-scale dairy farms in urban and peri-urban areas of Bishoftu town and
To assess potential management risk factors associated with calf mortality.
Calf
Calf refers to the age group of young cattle from birth to six or nine months of age (West, et al. [16]). Elsewhere it was defined as cattle up to six months of age after which in natural circumstances, it might be expected to be self-sufficient (Webster, et al. [17]). Calves have some special features in their body system that have significance in disease occurrence and accordingly require special attention in management. Those that have particular importance are the poorly developed defense mechanism and a dynamic digestive system that has to evolve from milk digestion to solid feed digestion. These make calves particularly susceptible to disease. In the digestive system of newborn calves, there are certain alterations. There is a delay in acid secretion from the stomach and in the development of pancreatic function; thus acid and trypsin digestion of protein is not started. The new born calves have also specialized intestinal epithelium capable of engulfing soluble protein, which will disappear within 24 hours after birth (Cunningham, et al. [18]). All these help calves to absorb intact immunoglobulin from the colostrum. Pre-weaned calves have a physiologically mono gastric stomach. The liquid feed flows through the closed esophageal groove directly into the abomasum, thus bypassing the fore-stomach. For new-born calves, it is most important that closures occurs prior to feeding, so that the liquid feed will be prevented from entering the rumen and causing digestive disturbance (Blowey, et al. [19]). Other chemical and physical components of the digestive system develop with age as the calf starts feeding on different feeds (Heinrichs and Radostits, et al. [2]).
Management of the Newborn Calf
The major factors which may predispose young farm animals to infection include: insufficient or no colostral immunity, overcrowding and poor hygiene which increase the transmission of the organism, naïve immune system in neonates and stress factors such as cold ambient temperature and frequent mixing of animals (Quinn, et al. [20]), calf nutrition and calf vaccination status (Smith, et al. [21]). Management of calves is essential in approaching the problem of neonatal death loss and because of infectious agents are almost always present at some exposure level, under lying is to minimize the level of pathogen exposure and stress on the calf (Smith, et al. [21]). In well managed dairy herd calf mortality usually does not exceed 5% from birth to 30 days (Radostitis et al. [22]). The first weeks of life are critical to the growth and long term performance in a dairy calf (Smith, et al. [21]). The emphasis is on insuring that the new born are born in a clean environment as well as barns, confinement pens and paddocks used as parturition areas must be clean and preferably have been left several days before the pregnant cow placed (Radostitis et al. [22]).
Importance of Colostrum to New Born Calves
It is generally considered that the new born calf should receive 4 L of colostrum in the first 12 h, although up to 6 L is often recommended for the first day (University of Sydney, [23]). Calves are born with no immunity to disease. Until they can develop their own natural ability to resist disease through exposure to the disease organisms in their surroundings, they depend entirely on the passive immunity acquired by drinking colostrum from their dam. Colostrum is the thick; creamy-yellow sticky milk first produced by cows initially following calving and contains the antibodies necessary to transfer immunity on to their calves. It is essentially milk reinforced with blood proteins and vitamins. It has more than twice the level of total solids than in whole milk, through boosted levels of protein and electrolytes. It also contains a chemical allowing newborn calves to use their own fat reserves to immediately provide additional energy (Moran, et al. [24]). Many studies demonstrated the importance of high levels of serum immunoglobulin in reducing the risk of morbidity and mortality in calves (Gebremdhin, et al. [14,25,26]).
Economic Importance of Calf Mortality
Diseases of the new born calf and neonatal calf mortality are major causes of economic losses in livestock production. As (Wudu, et al. [8,14]) we conclude in their study, the magnitude of calf morbidity and mortality were much higher than the economically tolerable levels and could affect the productivity of dairy farms through mainly decreasing the availability of replacement stock. Neonatal calf mortality in the first month of age is accounted to be 80-85% of the total mortality and is particularly high in the third week of life (Singh, et al. [27]). A dairy farm management system should employ a strategy that will reduce calf mortality and improve calf performance by controlling diseases. In good management practice, the annual mortality of calves less than one months of age can be reduced to below 3-5% and the first calving age at around 24 months (Heinrichs and Radostits, et al. [2]).
Calf Diarrhea
Calf diarrhea is the commonest disease in young calves and is the greatest single cause of death (Bekele, et al. [25,28]). Calf diarrhea was found to be the predominant calf health problem with an incidence rate of 42.9% (Wudu, et al. [8]). Diarrhea can be due to infectious (viruses, bacteria, and protozoa) and noninfectious factors. Co-infection is common. Ten different pathogens are recognized as either major (rotavirus, coronavirus, Bovine Diarrhea virus, Salmonella spp, E.coli, Clostridium perfringens, and Cryptosporidium parvum) or emerging (bovine caliciviruses and bovine torovirus) (Cho, et al. [29]) Neonatal calf diarrhea remains the most common cause of morbidity and mortality in pre-weaned dairy calves worldwide. This complex disease can be triggered by both infectious and noninfectious causes. The four most important entero pathogens leading to neonatal dairy calf diarrhea are Escherichia coli, rotavirus and coronavirus, and Cryptosporidium parvum (Meganck, et al. [30]).
Bovine Corona Virus
Bovine coronavirus is a component of the acute diarrheal complex of neonatal calves, a widespread and serious economic problem in dairy and beef operations. The disease occurs in farm and ranch calves 1 day to 3 or more weeks old (Timoney, et al. [31]).
Bovine Rota Virus
Disease caused by rotavirus is usually seen only in young animals, 1 to 8 weeks of age, but only rarely during the first week of life. Infection by Rotavirus leads to the destruction of epithelial cells at the apex of villi in the small intestine. With the virulent strain of Rotavirus, the loss of enterocytes exceeds the ability of the intestinal crypts to replicate; hence, the villi height is reduced with a consequent decrease in intestinal absorptive area and intestinal digestive activity leading to diarrhea (Aiello, et al. [32]) (Table 1).
Escherichia Coli
All calves become colonized within a few hours of birth with many varied strains of E. coli. This constantly changing population of the organism inhabits the calf’s intestine for life and is entirely normal and healthy (Quinn et al., 2002). Some strains of E. coli have the ability to adhere to the intestinal wall and produce toxins that cause scours. An example of this E. coli K99, which is referred to as an entero toxigenic E. coli K99 (ETEC). This strain is capable of causing disease in calves of less than one week of age (Abraham, et al. [33]).
Salmonellosis
The organisms Salmonella Dublin or Salmonella typhimurium are the main cause of salmonellosis in calves. Salmonella typhimurium DT104 has been recognized as highly pathogenic to calves, resulting in a high incidence of mortality, and has a wide range of antibiotic resistance, and is capable of rapidly developing new resistance patterns (Bernadette, et al. [34]) and others Salmonella enterica serovar heidelberg (Salmonella Heidelberg) cause salmonellosis in calves (Wudu, et al. [8]).
Cryptosporidiosis
Cryptosporidium parvum is the most common species found in calves and man, and it is transmitted readily to several newborn species of mammals by the fecal oral route (Scott et al., 1995). Infection with Cryptosporidium is more commonly reported in calves less than 1 month of age, and affected calves may shed large numbers of infective oocytes in the face. While infection is generally self-limiting, fatalities associated with Cryptosporidiosis have been reported (Trotz-Williams, et al. [35]).
Calf Pneumonia
Although it can affect pre-weaned calves, this is the most common of all diseases of weaned calves and causes the highest loss in this age group, both in terms of mortality and reduced growth rates and accounts for about 15% of calf mortality from birth to 6 months of age (Heinrichs and Radostits, et al. [2]). Calf pneumonia is a multiple etiology syndrome that is caused by one or more of a whole range of organisms, including bacteria (like Pasteurella multocida, P. haemolytica, Hemophilus somnus, Actinomyces pyogenes), viruses (like Respiratory syncytial virus, Parainfulenza virus type 3 etc.) and Mycoplasma (M. bovis, M. dispar) (Sivula, et al. [5,6]). Environmental factors like inadequate ventilation are essential in the occurrence of calf pneumonia. The disease is characterized by clear nasal discharge and raised temperature in early stage and coughing and faster breathing rate later. Some acute outbreaks can occur with fatalities before any significant coughing has been heard (Blowey, et al. [19]).
Navel Ill (Omphalitis)
Localized inflammation or infection of the contents of the umbilical cord external to the body wall is referred to navel ill (omphalitis). Omphalophlebitis, omphaloarthritis, and urachitis are terms used to further describing the extension of inflammation or infection from the external umbilicus to the intra-abdominal segment of the umbilical vein, umbilical arteries, and urachus respectively (Kasari, et al. [7]). The most frequently isolated pathogens from navel ills are E. coli, Proteus spp., Staph. spp., Bacteroides spp, F. necrophorum, and Klebsiella spp. Novel ill can lead to septicemia, arthritis, and fever, which cause the failed transfer of passive immunity. Prevention of navel ill is based on good maternity hygiene, reducing calf residency time in unhygienic calving pens, ensuring adequate early intake of good quality colostrum and navel antisepsis (Lorenz, et al. [36]).
Bloat
Abomasal or ruminal bloat is an abnormal accumulation of gas in the calf stomach. This is usually caused by abnormal fermentation of milk when the calf is allowed to drink large quantity intervals. However, other cause of bloat also exists if the bloating is severe; the pressure can interfere with the calf ability to breath, resulting in death. Treatment should be initiated immediately by release using a stomach tubes, trocure or large needles. The diet and feeding management should be evaluated to prevent the condition (Majak, et al. [37]).
Sudden Death Syndrome
Usually in calve 3 to 8 weeks age; this is caused by Clostridium perfringens bacteria that are found naturally in the soil and in the gut of normal calves. Clostrodial organisms multiplies rapidly under conditions when calves are over feed, grain, and milk ,producing powerful enterotoxins that damage blood vessels, the brain, and other tissues and causing sudden death. The same protection may be provided by vaccinating dry caws with colostrum type C and D toxoids at approximately 6 weak, 3 weak prior to calving. This will increase the concentration of imminoglobine against those specific Clostridium organisms in the colostrum, providing increased resistance to the disease in the calf (senger, et al. [38]).
Other Causes of Mortality
Other diseases in calves including arthritis, parasitic gastroenteritis, and parasitic pneumonia in grazing calves; arthropod parasites and nutritional diseases (like inadequate intake of energy, protein, vitamins, and minerals) are also reported (Heinrichs and Radostits, et al. [2]).
A range 15 to 25% pre-weaning calf mortality is typical on many tropical dairy farms. It is often as high as 50% indicating very poor calf management (Radostits, et al. [22]). This contrasts with US findings of less than 8% mortality from birth to 6 months while surveys of Australian farmers report only 3% losses (Moran, et al. [3]). One study on the prevalence and incidence of calf morbidity and mortality and associated risk factors was conducted in Hawassa town. The study shows the crude mortality rate was 9.3% (Bekele, et al. [28]). The overall incidence of crude mortality found in (Wudu, et al. [8]) study was 18.0%.
Determinants of Dairy Calf Mortality
Major diseases in dairy calves have multifactorial etiology, resulting from interactions between the calf, infectious agents, management and environmental factors.
Calf Factors
Age: Age of calf is the most important factor affecting mortality. Approximately 75% of mortality in dairy animals less than one year of age, occurs in the first month of their life (Heinrichs and Radostits, 2001). It was the only risk factor significantly associated with the risk of mortality in all calves (weaned and un-weaned). In all cases (mortality, morbidity, and calf diarrhea), younger calves under three months of age were at higher risk compared to older calves (Wudu, et al. [8]).
Breed: Differences in the susceptibility of calves to diseases are often observed among different breeds. Taurine breeds and their crosses are generally more susceptible to diseases in tropical clim ificant variations in calf mortality rate (Mansour, et al. [39]).
Environmental and Manage Mental Risk Factors
ates. In one study in Ethiopian highlands, a higher mortality rate (62%) was recorded in 87% Friesian x-Boran crosses than 75% Friesian x-Boran crosses (32%) (Gryseels and de Boodet, et al. [40,41]), also found higher calf mortality in exotic breeds than locals. In contrast to this study, the breed of calf showed no signs.
Feeding Management
Calf healthcare is generally dependent to a great extent on management and hygiene practices, as the majority of calf disease arise as a result of poor management (Roy, et al. [42]). Ingestion and absorption of enough quantity and quality of colostrum is a critical determinant for the health and survival of neonatal calves. Calves, which do not receive adequate colostrum, are shown to have a higher overall death rate and are more likely to develop scouring and pneumonia, even at two to three months old (Blowey, et al. [19,43]) found that calves with inadequate blood colostral immunoglobulin concentration in 24 hours of birth were at greater risk of neonatal morbidity and mortality, pre-weaning morbidity and morbidity and respiratory problems while in a feedlot. Several reports have shown the importance of colostrum in relation to the risk of calf mortality and morbidity (Edwards, et al. [4,44,45]). This problem can be alleviated to some extent by assisted natural sucking, but this can fail because not all calves requiring assistance are detected. An alternate approach is to milk 2 L of colostrum from the dam bottle, feed each calf as soon after birth as possible, then leave the calf with the cow for 24 hours, and allow it to suck voluntarily. While this will not be as effective as a system based entirely on artificial feeding of the selected colostrums, it is an app roach that is suitable for the smaller dairy farms. The chance of calves surviving the first few weeks of life is generally reduced if they do not ingest and absorb these antibodies into their blood system. It takes far fewer disease organisms to cause disease outbreaks in such calves than if they can acquire immunity from their dam. Calves without adequate passive immunity are four times more likely to die and twice as likely to suffer disease as those with it (Moran, et al. [24]). As (Wudu, et al. [8]) reported in his survey of calf morbidity and mortality in dairy farms in Bishoftu a close relationship between calf mortality and when calves drink their first colostrum. If later than 6 hours after birth, calves had higher morbidity and mortality than if they consumed it early in life (Table 2).
Calf Housing
Housing has a significant effect on the health of the calves, with unclean barns predisposing calves to pneumonia (Wudu, et al. [8]). On most dairy farms, calves are taken from the maternity area soon after birth and placed in the calf-rearing barn. This is due to stressed and calving cows shed bacteria at a much higher level than their unstressed peers. Pre-weaned calves that share the housing facility with adult cows, sick cows, or recently weaned calves have a much greater risk of exposure to pneumonia and fecal pathogens. Calf -to-calf contact increases the number of pathogens in the environment. This is rarely the most important risk factor, but distancing calves or creating barriers that prevent cross-suckling, licking, or manure contact can reduce the rate of exposure (McGuirk, et al. [46]).
Herd Size
A marked increase in population density commonly results in an increase in the incidence of infectious diseases. Different studies reported significantly lower calf mortality in dairies having small herds than large or medium herd size (Bruning-Fann and Kaneene, 1992). (Garber, et al. [47]) found a higher prevalence of Cryptosporidium associated with larger herd size in dairy farms. Herd size by itself has not a biological effect on calf health; rather, it may be a measurement of other factors like time available to observe and care for calves. Other possible reason for the apparent association between herd size and calf mortality could be that in case of small herd sizes, enough time may elapse between successive births, which will reduce the concentration of infectious agents in the calf-rearing environment.
Some authors have reported calf mortality proportions in different parts of Ethiopia. Those information on calf mortality in market-oriented small holder dairy farms available are summarized in the following table.
Study Area
The study was conducted at Bishoftu Town. Bishoftu Town is located 45 km South East of Addis Ababa, capital of Ethiopia. It lays 90N latitude and 400E longitude at an altitude of 1850m above sea level. The rainfall is bimodal. It receives an annual rainfall of 1151.6mm of which 84% is received during the long rainy season covering June to September and the remaining in the short rainy season extending from March to May. The dry season extends from October to February. The mean maximum and minimum temperature of the area is 360C and 140C respectively, and mean relative humidity is 61.3% (NMSA [48]).
Study Population
The sampling units for the study were crossbreed dairy calves of up to six months of age. All calves from small holder dairy farms managed under intensive and semi- intensive system in urban and peri-urban constituted the study population. There were 150 smallholder dairy farms with a herd size of eleven cows in average which registered in the urban agricultural development office of Bishoftu town.
Study Design
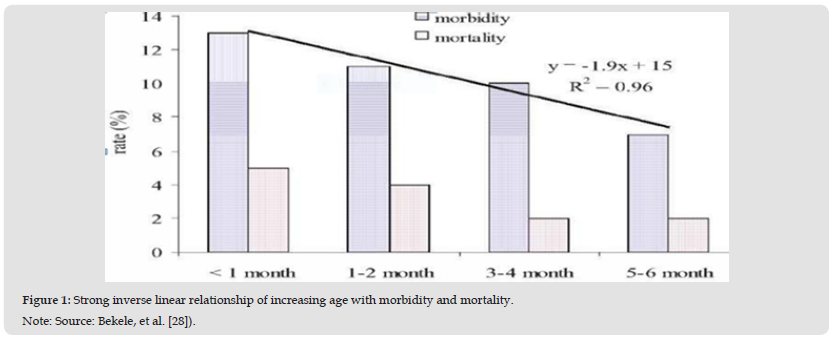
The sampling units for the study were crossbreed dairy calves of up to six months of age. All calves from small holder dairy farms managed under intensive and semi- intensive system in urban and peri-urban constituted the study population. There were 150 smallholder dairy farms with a herd size of eleven cows in average which registered in the urban agricultural development office of Bishoftu town. The study was a longitudinal prospective observational study that extended for six months from November, 2016 to April 2017. The sampling units (calves) were identified individually and observed throughout the study period in a two week interval (Figure 1).
Figure 1 Strong inverse linear relationship of increasing age with morbidity and mortality. Note: Source: Bekele, et al. [28]).
Sampling Procedure and Sample Size
First, dairy cattle were sampled by cluster sampling technique from all dairy farms of the town. A sampling frame, i.e., the list of dairy farms, was acquired from the urban agricultural development office of Bishoftu town at the beginning of the study. Dairy farms were selected from the list using a random sampling procedure to ensure the selection of proportional and representative sampling of dairy farms from both urban and per-urban sectors. Accordingly, 60 small holder dairy farms were visited on a cluster basis. In these selected farms, all calves less than two months and pregnant caw >seven months were included in the observation. Initially, the sample size using a simple random sampling method was determined at 11.6% expected calf mortality rate for Bishoftu (Gebremedhin, et al. [9]) for large dairy farms, 95% confidence level and 5% required absolute precision by using a mathematical formula (Martin, et al. [49]). The adjustment for cluster sampling using cluster size and intra-cluster correlation was made as follows.
N=n[1+((m-1)*p)]
Where,
N=sample size for cluster sampling
n= sample size calculated for simple random sampling
m=average cluster size
p= intra cluster correlation
However, in this particular case, as the average herd (cluster) sizes (calves per small-scale dairy farm) were small, the effect of intra-cluster correlation would be small and “N” will be very close to “n”. So the sample size determined by simple random sampling was taken to be the sample size for the study (Martin et al., 1978).
n=((Zα/2 )^2 (p(1-p))^2)/Δ^2
n=sample size
Zα;∕2= confidence level
P=expected prevalence
∆=precision level
Accordingly, data on 158 calves of new born and born before two months before the start of the study were included. When a selected farm did not have a calf or calves (having calves under two months of age, a pregnant caw > seven month, or the owner is not interested eligible for the study and it was replaced by another farm.
Observation of dairy farms for calf mortality was carried out for four months. For this study, calf was defined as young cattle less than six months of age and mortality was defined as death of calves above the age of 24 hours. For the observation, all calves in the selected farms that were under two months of age at the beginning of the follow-up period and those born in the subsequent months of follow-up were included. This way, each calf was observed for four months unless censured due to death. Individual records were prepared when a calf joined the study cohort. These were used to record genealogy of the calf, events surrounding the birth of the calf, routine management practices applied to the calf, and health problem incidents that happened during the observation. In the actual observation work, calves were regularly visited every two weeks. During this period, observations and in-depth interviews with farm owners or workers were made on different aspects associated with calf health problems and potential animal and management risk factors including: calf barn, sex and age of the calf, time of colostrum provision, milk supplementation after separation, naval disinfection, veterinary service, the amount of colostrum provision, age of separation from the dam, occurrence of calf mortalities and the general herd management aspect of the farm (Figure 2).
Describing Mortality Problems
As animals in this longitudinal study were recruited at different times and were followed for different periods of time, and thus incident density (true rate) was used in describing death occurrence. Incident density was calculated by dividing the number of deaths by the number of calf weeks at risk. Number of calf weeks at risk was found by adding the number of weeks at risk of obtaining a new death in each calf in the study period.
Statistical analysis
Questionnaire data were recorded on Microsoft Excel sheets and analyzed using SPSS version 20. Descriptive statistics was employed to determine calf mortality rate. First, the association of individual risk factors with a death variable was screened by univariable logistic regression. Those variables with p-value of less than 0.25 were selected forward for multivariable logistic regression. For all factors, a P-value of less than 0.05 was set to be the indication of association with death of calves.
Incident Rate
Observation of 158 dairy calves for mortality cases was conducted for 6 months. The results of this study revealed that the cumulative mortality proportion in the first 6 months of calf hood was 8.2%. Among the causes of calf death recorded in this study, calf diarrhea was the leading cause of calf mortality with cause- specific mortality proportion of 3.2%, followed by septicemia (1.3%), pneumonia (1.3%) and nonspecific causes (1.3%). The mortality proportion of other causes of death (bloat and accident) recorded in this study was equivalent to cause-specific mortality proportion of (0.63%). The calf death rate or incident rate was 0.0055/calf week. The average for the occurrence of mortality incidents was 10 weeks. Proportionally, the highest mortality incidents occurred in the first week of life, in which 61.5% of the total cases of mortality occurred. Again, 76.9 per cent of the total cases of crude mortality occurred in the first month of age and 100 per cent of the total cases of crude mortality occurred in the first three months of age.
Association of Potential Risk Variables with Incidence of Calf Mortality
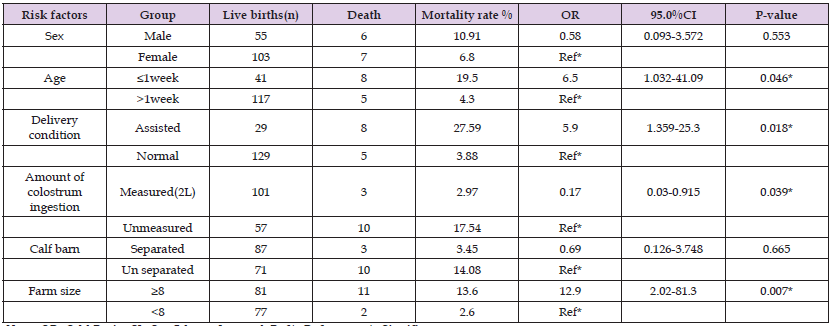
A total of eleven different potential risk factors (sex, age, navel disinfection, time of colostrum ingestion, milk supplementation after separation from the dam, delivery condition, and amount of colostrum ingestion, age of separation from the dam, calf barn, and farm size and management system of the farm) were investigated for their potential association with the occurrence of crude calf mortality. Among the risk factors assessed, age of the calf (OR=6.5, P= 0.046), delivery condition (OR=5.9, P=0.018), the amount of colostrum ingestion ( OR=0.17, P = 0.039) and farm size (OR = 12.9, P = 0.007) were found to be significantly associated with the death of calves while the other risk factors; separation of calf house from the farm and sex were not found to be significantly associated to the death of calves (Table 3).
Table 3: Multivariable logistic regression model output for potential risk factors of calf mortality.
Description of Household and Livelihood Characteristics of the Farmers Based on Questionnaires and Observation
The majority of the farm owners were male (61.7%) and the rest (38.3%) were female. 48.7% of the age of the owners was between 31-50 years while 26.6%, 16.7%, and 8.3% were above 51years, 25-30 years, and less than 25 years respectively. As far as household literacy is concerned; (16.7%), of the farm owners were illiterate 30% attended primary school 33.3% completed secondary school and diploma, and 20% were college graduates. The average herd size per household of the dairy cattle in the study area was 11 and ranged from 5 to 17 heads of cattle. The average number of cross-breed calves per household was 2. About 61.7% of the owners had farm experience more than ten years, while the rest 28.3% and 10% had 5-10 years and less than 5 years, respectively. About 86.7% of the farm owners have no calving pen. All owners use artificial insemination (AI) for breeding and 81.7% of the owners use dairy farms as a secondary source of income, where as 18.3% of the owners mainly depend on the farm for livelihood. As the owner replied that hypocalcaemia (36.7%), mastitis disease (33.3%), bloat (15%), pasteurellosis (10%), and others (5%) were the common diseases affecting dairy farms. Majority of the farmers (88%) had knowledge of the advantage of colostrum over ordinary milk and all of them did know the optimum time to feed colostrum to calves and provide milk supplementation after separation from the dam but the majority of them did not know the amount of colostrum needed for the new born calf within 24 hours. None of them practice naval disinfection and all of them call private veterinary practitioners whenever facing the health problems of animals. From farmers that mentioned calf health problems as a problem in dairy production, the majority of them (50%) complained diarrhea as a major cause of calf morbidity and mortality. Majority of the owners sold male calves at young age while they kept female calves for production purposes. The housing systems of the major dairy owners were loose and no separation between calf and cattle houses. Most of the owners employ a person who keep their cattle and care for their calves.
In the present observation, the overall mortality percentage of live born calves up to four months of age on small holder dairy farms is found to be 8.2% which is considered as high. Different authors reported a wider range of calf mortality rates in Ethiopia. Reported calf mortality varies widely depending on many factors, some of which may be specific to the particular population being studied. The finding of the present study is in agreement with 9.3% and 10.2% mortality proportions reported by (Bekele, et al. [28,50]). In contrast, the present finding was lower than the previous mortality reports in different parts of the country; 18.0% in Debrezeit (Wudu, et al. [8]), 16-22% (up to 6 months age) in Wolaita Sodo (Assefa, et al. [51]), and 30.7% in Bahir Dar and Gozamen districts of Amhara Region (Ferede et al., 2014). The relatively less mortality rate in this study compared with the findings of (Wudu, et al. [8]) in Debrezeit is probably due to the current better access to veterinary services in towns and their suburbs. In addition, the time elapsed for this investigation was lower than the previous one. In the present study, the major causes of calf mortality identified were diarrhea, pneumonia, septicemia, bloat, accident, and nonspecific causes. However, calf diarrhea was found to be the dominant cause of calf mortality with cause - specific mortality percentage of 3.1%. This finding is in agreement with the reports of (Lemma, et al. [8,26,52-54]), in Ethiopia, and many other studies elsewhere, which reported diarrhea as the first most important health problems causing calf death (Olsson, et al. [6,15,55,56]). Calf diarrhea as a leading health problem in growing dairy calves is a common finding. The high incidence of mortality in this study suggests the significant improvement of farm management and failure of adequate immunoglobulin due to the majority of the farm owners having the wrong perception that feeding a large amount of colostrum causes diarrhea in calves.
The lower mortality rate recorded for pneumonia in this study is with the studies conducted by (Rao and Nagarecenkar, (9,57-59]), which found pneumonia as the leading cause of calf mortality. The lower mortality incidence rate recorded for pneumonia in this study could be due to most of the owners using loose housing systems which provides good ventilation. This is supported by (Blowey, et al. [9]) who indicated that low calf mortality due to pneumonia has occurred in calves kept in good ventilated houses. High proportion of mortality due to unknown cause which is characterized by sudden death needs a well-designed study. This investigation showed that mortality was higher during the first week of birth (OR=6.5; P≤ 0.046) and decreased with increasing age. Age was found to be the most important calf factor affecting their mortality. Among dead calves, 61.5% of deaths occurred in the age of less than 1 week. In this study, younger calves under the first months of age were at higher risk of mortality. Similar age pattern of mortality has been reported by previous studies (Wudu, et al. [8,28]). The higher risk of mortality in young calves observed in this study suggests the need of more careful management for very young calves. Other risk factors were assessed to determine the magnitude of their association with the occurrence of dairy calf mortality delivery condition. Mortality in calves delivered by assisting was higher compared to calves delivered normally (OR=5.9, p=0.018). The odds ratio (5.9), we estimated that the odds of death for cross-bred calves born assisted were around 6 times higher than calves delivered normally. When calves are suffering from pain after calving assistance, they become weak to stand and suckle. (Murray, et al. [60]) found that calves born following dystocia were more acidotic and took longer to attain sternal recumbence and stand, compared to calves born normally and (Uigley, et al. [61]) noted that new born calves stressed due to dystocia are weak enough to adapt to life in the external environment. This stress to the calves probably reduced the immunoglobulin absorption efficiency as well as delayed or decreased intake of colostrum. Hence, the longer the calves are without adequate colostrum Ig, the more opportunity for the pathogens that provoke diarrhea to invade the gut.
The logistic regression analysis of mortality in dairy calves with respect to the amount of colostrum ingestion revealed that the highest mortality rate was observed in calves which took inadequate and unmeasured colostrum (OR=0.17; p=0.039). Hence, this study agrees with the theory which says that absorption of enough quantity and quality of colostrum is a critical determinant for the health and survival of neonatal calves. Calves which did not receive adequate colostrum are shown to have a higher overall death rates. To ensure adequate protection against disease, calves rely on the intake of an adequate amount of quality colostrum within a few hours of birth (Arthington, et al. [62]). In this study, significantly (OR=12.9, P=0.007) high mortality of dairy calves occurred in farms where greater than eight animals were kept. This finding is supported by (Frank and Kaneene, et al. [28,62-65]), Herd size has been positively associated with an increased incidence of calf diarrhea, which is one of the leading causes of mortality. This may be due to overstocking or this difference might be owners who kept less than eight animals have given sufficient time for nursing the sick calves which might be the reason of lower mortality. Against to the present finding, other studies conducted in Waliata Sodo town on dairy calf morbidity and mortality (Assefa, et al. [51]) revealed that calf mortalities were high in dairy farms when less than ten animals kept.
The present study has estimated that the calf mortality proportion of 8.2% is higher than the economically tolerable level in dairy cattle. Mortality was higher at the lower age of calves, in the first months of life. It has also found that age of calves, unmeasured amount of colostrum, feeding and delivery condition, and farm size were important risk factors associated with calf mortality. This is a great hindrance to improve smallholder dairy production and productivity in the study area. The leading disease condition associated with mortality was found to be diarrhea. Known fact that the observed farms raise their own replacement stock and have small herd size, it is recommended that special emphasis should be given naval disinfection of newborn calves, having confinement pens and paddocks used as parturition areas, the amount of colostrum feeding, implementation of proper management as the farm size increases and especial care management of first week age of calves were found important to minimize calf mortality. Moreover, further investigation is suggested to identify the specific causative agents incriminating for calf mortality in dairy farms of the study area.
Since the research was undertaken by collecting data through enter viewing the owner of the farm it did not abuse any animal welfare protocols. All procedure of data collection was carried out in accordance with relevant guidelines. Before data collection, the objective of the study and types of data collected were clearly explained for the owner of the farms (farmers), then those owner who had willingness to cooperate and participate in the study were included. Therefore, his research studies comply with international guidelines.
I declare that the authors have no competing interests, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
I am writing this to inform you that, I am from lower income economy country (Ethiopia) and I haven any financially supporting body (Funding Institution) who make payment for Article processing charge. Hence, since I am student I would like to ask your financial support in order to cover or cancel APC and publish this article without any fee.
Since I want to work with the scientific and research community, the data underlying the findings of a paper should be publicly available wherever possible as open as possible. I therefore firmly support and endorse the Findability, Reusability and Accessibility of this article. So that, I prefer to deposit the data in a public repository that meets appropriate standards of archiving, citation and curation.


