Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Quan Ren1*, Mengshuang Qiang2, Dandan Liu1, Weili Yu2, Ying Zhang2, Yemeng Zhang2 and Jie Sun1
Received: February 03, 2023; Published: February 14, 2023
*Corresponding author: Quan Ren, Department of Anesthesiology, Zhongda Hospital, Southeast University, 87 Dingjiaqiao RD, Zhongda Hospital, Nanjing, JS 210009, China
DOI: 10.26717/BJSTR.2023.48.007687
Background and Objective: China domestic alfentanil hydrochloride (Yichang Human Well Pharmaceutical Co. Ltd., Yichang, China) is the first alfentanil pharmaceutical preparations produced in China, which has been approved for clinically use by the China Food and Drug Administration since Match, 2020. Its clinical pharmacological characteristic needs to be validated in clinical practice. The trial was conducted to compare the efficiency and adverse effects of China domestic alfentanil hydrochloride with sufentanil citrate (positive control) in elderly patients during perioperative period.
Methods: This was a single-centered, randomized, triple-blinded, positive controlled, parallel grouped, non-inferiority trial that was conducted in a Class A tertiary hospital in China from December 2020 to Match 2022. 248 relatively healthy elderly patients were randomly allocated to receive equivalent alfentanil or sufentanil (the potency ratio 1:65) in general anesthesia of relatively short surgeries. Differences of incidence and severity of cough after injection of the 2 drugs were set to be the primary endpoint investigated. Other measurements such as muscle rigidity, nausea and vomiting, mean blood pressure and heart rate reactions, skin pruritus, and urinary retention were also compared.
Results: The incidence of cough after venous injection of alfentanil was much lower than that of sufentanil (11.66% vs 21.88%, 95% CI of incidence difference was 0.8% ~ 19.7%, P = 0.042). Noninferiority test (margin = 5%) revealed valid in favor to alfentanil, and also reached superior level. As to postoperative nausea and vomiting (PONV), the incidence was also significantly lower in alfentanil group than in sufentanil group (8% vs 21.1%, P = 0.0066), and severity of mild and moderate were also lower in alfentanil group than in sufentanil group (P = 0.0358). Mean blood pressure and heart rate reactions were similar after applying the two drugs.
Conclusion: The data of this study show that, the efficiency of China domestic alfentanil is similar to that of sufentanil. Alfentanil is superior to sufentanil in adverse effects of cough and PONV inducing effects in relatively short elderly surgeries.
Keywords: Elderly Adults; Opioid Safety; Cough Response; PONV; Non-Inferiority Study; RCT Design
Opioids is the key analgesics in general anesthesia practice for their stronge and reliable pain release effect. At the same time, the adverse effects of opioids such as respiratory depression, cough and muscle rigidity after quick intravenous injection, PONV, pruritus, and urine retention are also apparent, which deserve attention of clinicians. Alfentanil, first synthesized by Dr. Paul Janssen, Belgium, in 1976, is a newer generation of synthetic powerful opioid analgesics than fentanyl, and sufentanil [1]. Alfentanil has been widely used in Europe, Japan, and America for decades. Several clinical guidelines [2-4] have recommended alfentanil for day surgery, procedure sedation and analgesia, and flexible bronchoscopy in light of its shorter action time, high therapeutic margin of safety (LD50/ED50) [5], lower incidence of side effects, such as coughing, hypotension and bradycardia, PONV, and respiratory depression. China domestic alfentanil hydrochloride (Yichang Human Well Pharmaceutical Co. Ltd., Yichang, China) is the first alfentanil pharmaceutical preparations produced in China, which has been approved for clinically use by the China Food and Drug Administration since Match, 2020. Its clinical pharmacological characteristic needs to be validated in clinical practice. We are interested in its advertised merit of less side effects compared with its congeneric opioids in elderly. So, we conduct a triple-blind, 2-arm randomized, positive controlled trial in elderly patients to test the efficiency and adverse effects of China domestic alfentanil hydrochloride (later referred to as alfentanil) in comparation with those of equivalent China domestic sufentanil citrate (later referred to as sufantanil).
Study Design and Patients
This study was a single-centered, prospective, randomized,positive-controlled, triple-blind, non-inferiority trial. Ethical approval for this study (Ethical Committee No 2020ZDSYLL217-P01) was provided by the ICE for Clinical Research of Zhongda Hospital Affiliated to Southeast University, Nanjing, China (Chairperson Prof H. Tang) on 6 November 2020. Every patient (participant) or his/her agent needed to sign the written informed consent before he/she was recruited into the study. From December 2020 to Match 2022, the Chinese patients who were hospitalized and scheduled to receive surgical therapy in Zhongda Hospital affiliated to Southeast University were scanned for eligibility of enrollment by one researcher the day before the scheduled surgery. The anesthetist in charge of the scheduled anesthesia talked with the potential participants and obtained the signed consent form of participating in the investigation to confirm the enrollment of participants. The patients’ inclusion criteria were age 65-85 years old; body weight (kg) within (height(cm) - 100) ✕ (1±15%); American Society of Anesthesiologists (ASA) classification of I or II; and the expected operation time less than 2 hours. The patients’ exclusion criteria were long-term or short-term medication with drugs such as opioids, NSAIDs, sedatives, antidepressants, anti-pruritus, and monoamine oxidase inhibitor 24 hours before the scheduled operation; history of opioid allergy or abnormal surgical anesthesia recovery; with esophageal reflux disease; uncontrolled hypertension before the scheduled surgery; involved in other drug trials within three months. Reasons for shedding cases were cancellation of surgery; more than 2.5 hours of operation time; and failure of follow-up due to early discharge of participants on the first day after surgery.
Randomization, Blinding and Interventions
Upon enrollment every participant was given an experiment number in a time sequence manner and a corresponding random code through the random number table method. The experiment number and the corresponding random code for every participant were kept by a researcher who prepared the experiment drugs, alfentanil or sufentanil solution, but did not participate in preoperative interview, intraoperative management, data collection or postoperative follow-up. The experiment drugs were prepared according to the random code. Odd and even random codes correspond to alfentanil solution and sufentanil solution, respectively. All other researchers were blind to the random code and participants’ grouping. The alfentanil solution was prepared with domestic alfentanil hydrochloride injection (Yichang Human Well Pharmaceutical Co. Ltd., Yichang, China) and normal saline, the alfentanil density was 2 mg/6ml; correspondingly, the sufentanil solution as a positive control was prepared with sufentanil citrate injection (Yichang Human Well Pharmaceutical Co., Ltd., Yichang, China) and normal saline, the sufentanil density was 30 mcg/6ml. The alfentanil / sufentanil potency rate of approximate 1:65 was applied in this study. Unblinding took place at the end of data collection. The general anesthesia was carried out following the same protocol except for the experiment drugs, by registered anesthesiologists and their assistants who were blind to the patients’ random code and grouping. The assistants documented the experiment data. The postoperative follow-up evaluation was performed by a specific evaluator. Neither the participants nor the evaluators were aware of the participants’ grouping.
Protocol for General Anesthesia and Recovery
The participants were not given any drug before entering operation room. After entering the operation room, upper limb venous access and routine monitoring, i.e. electrocardiogram, pulse oxygen saturation, body temperature, invasive arterial blood pressure, and bispectral index (BIS), were established. During general anesthesia induction, midazolam 0.02mg/kg, the experiment drug 0.06ml/kg (equal to alfentanil 20 mcg/kg or sufentanil 3 mcg/kg) (completed in 5s), propofol 1 ~ 1.5mg/kg and rocuronium 0.6mg/kg were injected intravenously in a row. Anesthesia was maintained by continuous inhalation of 1% sevoflurane, intravenous infusion of 1% propofol to keep BIS value between 40 ~ 60, intravenous infusion of 5mg% remifentanil to keep mean blood pressure (MAP) in the normal range, and adding experiment drugs if necessary at the anesthesiologists’ discretion. Pressure of end tidal CO2 (PetCO2) was monitored continuously after patients lost consciousness and mask mechanical ventilation started and was kept between 35-45 mmHg through adjusting minute ventilation volume. Azasetron 10mg was given to prevent PONV 10 minutes before the end of the operation. No other analgesic drugs were used throughout the procedures. The obvious circulatory instabilities, such as bradycardia less than 50 bpm, hypotension less than 20% baseline blood pressure / or 90 mmHg systolic blood pressure, and hypertension more than 20% baseline blood pressure / or 160 mmHg systolic blood pressure was controlled with vasoactive drugs such as atropine, deoxyepinephrine, dopamine, urapidil or nicardipine at the anesthesiologists’ discretion. During the early postoperative period, the participants were cared and extubated at the recovery room according to the routine protocol of resuscitation by another anesthetist who was blind to the participants’ grouping. The patients were observed for another more than 30 minutes after extubation before sent back to the ward. In the recovery room, adverse events, such as the obvious circulatory instabilities, PONV, and severe pain complain were recorded and controlled with vasoactive drugs, metoclopramide, and oxycodone at the discretion of the anesthetist in the recovery room.
Outcomes and Measures
During the general anesthesia induction period, incidence and severity of cough and muscle rigidity, and vital signs, i.e. MAP and heart rate (HR), were recorded. Incidence of cough and muscle rigidity were observed before rocuronium injection. Cough severity was classified into mild (1-2 times), moderate (3-4 times), and severe (5 times or more). Muscle rigidity was classified into mild (high ventilation pressure and/or low PetCO2) and severe (no ventilation volume could be achieved until rocuronium injection). MAP and HR were recorded at 4 time points: immediate after entering operation room (T1), before induction (T2), immediate (T3) and 5 minutes (T4) after endotracheal intubation. During the intraoperative period, the consumptions of general anesthetics, i.e. propofol and remifentanil, and the incidence of adverse cardiovascular events (circulatory instability) were recorded. In the recovery room, the time to extubation were recorded. The Ramsay sedation scores of patients were assessed 30 minutes after extubation. The incidence of nausea and vomiting, and incidence of moderate and severe pain complain (pain visual analog score (VAS) more than 3/10) needed oxycodone treatment were recorded. In the postoperative day 1 and 2, the incidence of urinary retention and skin itching, the incidence and severity of PONV, and the pain VAS score were recorded and analyzed.Patients’ pain VAS score was divided into 1-10 points, 1-3 points were defined as mild, 4-6 points as moderate, 7-10 points as severe, respectively. PONV severity was defined as: 1-3 times per day was mild, 4-6 per day was moderate, and 7-10 times per day was severe. Ramsay's sedation scoring standard: 1 point, restless and irritable; 2 points, quiet cooperation; 3 points, sleepy, able to follow instructions; 4 points, sleep state, but can wake up; 5 points, sleep state, response to strong stimulation, slow response.
Sample Size and Statistical Power
The hypothesis of this study is that the equivalent China domestic alfentanil hydrochloride would be non-inferior to China domestic sufentanil citrate in term of adverse effects such as induction cough, muscle rigidity, or PONV. So, non-inferiority statistical method was used for sample size determination. Among the adverse effects, the incidence of cough caused by alfentanil and sufentanil reported in previous literature was the relatively low, 8% [6] and 15.8% [7], respectively. Thus, we chose incidence of cough as the primary outcome for analysis. Other measurements mentioned above were secondary outcomes. In order to detect the non-inferiority nature of alfentanil to sufentanil in term of cough incidence, the minimal whole sample size was estimated to be 124 in each group to provide 80% power (one-tailed 𝛼 of 0.025), presuming the non-inferiority margin 𝛿 = -5%, and drop-out rate to be 20%.
Statistical Analyzes
SPSS statistics 26.0 was used for all statistical analysis. Continuous variables of normal distribution are expressed as mean ± standard deviation (SD); non-normal variables were reported as median (interquartile range). Continuous variables such as demographics, baseline data evaluation, total amount of experimental drugs and unit time consumption of other general anesthetics were analyzed by t-test or nonparametric rank sum test. The analysis of categorical variables such as the incidence and severity of cough, the incidence of muscle rigidity, the incidence and severity of PONV, and the incidence of cardiovascular events were performed by chi square or Fisher test. When P< 0.05, the results were considered statistically significant.
Participants Flow
The study flow of participants is depicted in Figure 1. A total of 726 patients were screened for eligibility. 427 were excluded based on the inclusion protocol and exclusion protocol. 248 participants were enrolled into the study. It was disclosed after unblinding at the end of the data collection that 113 patients were allocated in alfentanil group (group A), 135 patients were in sufentanil group (group S). 231 participants completed the trial (103 in group A, and 128 in group S) for the anesthesia induction section; and 223 participants (100 in group A, and 123 in group S) also completed the rest of sections of the trials.
Demographic and Clinical Profiles of Participants
Demographic and clinical profiles of participants are displayed in Table 1. All the participants completed the experiments during induction period of anesthesia and were included into the analysis of effects of alfentanil and sufentanil during the induction period. The distribution of baseline variables was similar, and there was no significant difference in outcome indicators between the two groups (all P > 0.05). These results suggest that the 2 groups were comparable at baseline.
Table 1: Demograghic and clinical profiles of participants who only completed experiments of anesthesia induction section.
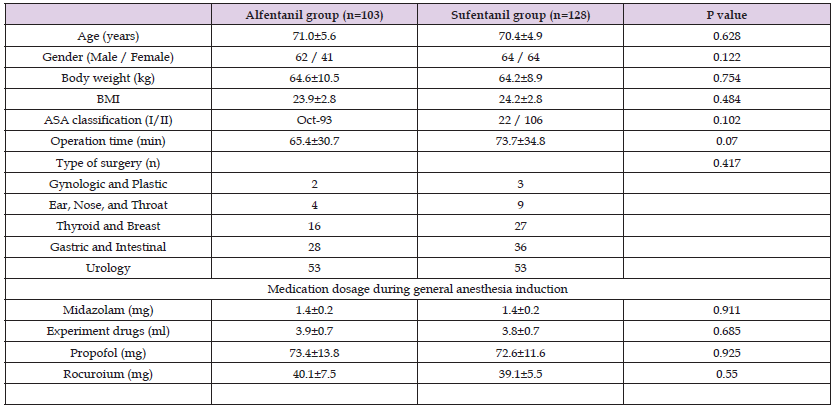
Note: BMI indicates body mass index; ASA indicates American Society of Anesthesiologists.
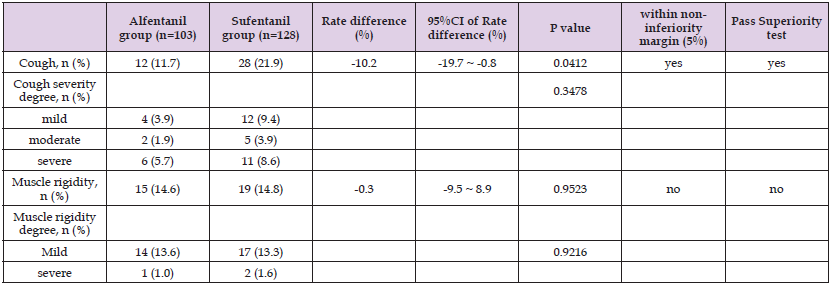
Table 2: Between-group differences of adverse effect incidences during anesthesia induction section.
Comparison of the Effects of Alfentanil and Sufentanil During Anesthesia Induction
As the primary outcome this study, the incidence of cough during the general anesthesia induction period was much lower in group A than in group S (11.66% vs 21.88%, P = 0.042), the rate difference was -10.22% (95%CI, -19.69% ~ -0.75%), which meets the non-inferiority (margin = 5%) hypothesis. Further, the result also reaches the superior level. (refer to Table 2.). These results indicate that alfentanil is superior to sufentanil regarding their cough inducing side effect. The incidences of muscle rigidity in the 2 groups were both near 15%, the rate difference is around 0, no statistical significance can be reached (refer to Table 2). The vital signs, i.e. MAP and HR, shifted significantly along the time curse in both the 2 groups, P < 0.01. But no significant difference was detected at each time point among the 2 groups, P > 0.05. (ref to Table 3, and Figure 2). These results indicate that alfentanil and sufentanil have similar side effects of muscle rigidity and similar effects on vital signs during induction of general anesthesia.
Figure 2 Heart rate during anesthesia induction period and Blood pressure during the anesthesia induction period.
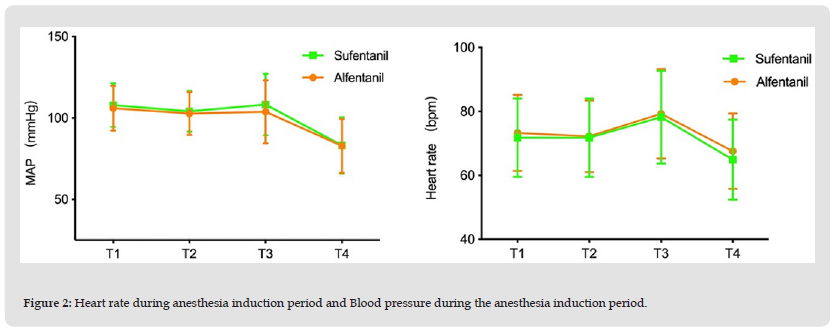
Table 3: Comparison of the vital signs between alfentanil and sufentanil groups during general anesthesia induction period.
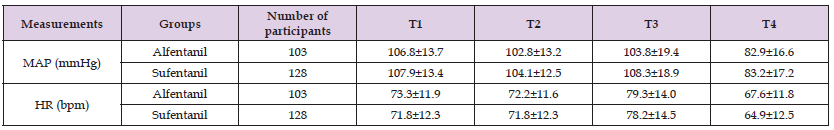
Note: Table 3 shows the vital signs, i.e. MAP and HR, of the 2 groups at different time points (T1 through T4) in the general anesthesia induction period. There are no significant differences between the 2 groups regarding the MAP or HR at all time points, P > 0.05. Repeated measure of 2-way ANOVA was applied for this statistic analysis. As to the influence of time, both MAP and HR show a time-dependent shift in the 2 groups, P < 0.01. MAP indicates mean blood pressure; HR indicates heart rate.
Comparison of the Effects of Alfentanil and Sufentanil During Intraoperative and Postoperative Period (Ref to Table 4.)
Since the data of participants who failed to complete or were excluded from experiment due to the exclusion criteria may affect the effects analysis, the intraoperative and postoperative effects of alfentanil and sufentanil are analyzed among the participants who completed the entire study. The demographic and clinical profiles of these participants are displayed in Table 5. In general, the distribution of baseline variables was similar between the 2 groups, except that the consumed volume of alfentanil in alfentanil group was more than that of sufentanil in sufentanil group (4.9±0.9 ml vs. 4.6±1.0 ml, P = 0.024), and the consumption of propofol per unit time in alfentanil group was greater than that in sufentanil group (2.9±1.0 mg/kg/h vs. 2.6±0.9 mg/kg/h, P = 0.027).
Comparison of Intraoperative Cardiovascular Events Between the Two Groups: Intraoperative cardiovascular event was defined as obvious circulatory instabilities in the context of BIS value between 40 and 60, such as bradycardia less than 50 bpm, hypotension less than 20% baseline blood pressure / or 90 mmHg systolic blood pressure, and hypertension more than 20% baseline blood pressure / or 160 mmHg systolic blood pressure that needed vasoactive drugs such as atropine, dopamine, deoxyepinephrine, urapidil, or nicardipine treatment. The incidences of intraoperative cardiovascular events of the two groups were both near 65%, the rate difference between 2 groups was 0.04%, P = 0.9949 (ref to Table 4.). These results suggest that alfentanil and sufentanil has similar effects on cardiovascular system in the operation.
Table 4: Between-group differences of adverse events incidences in intraoperative and postoperative period (including in the recovery room).
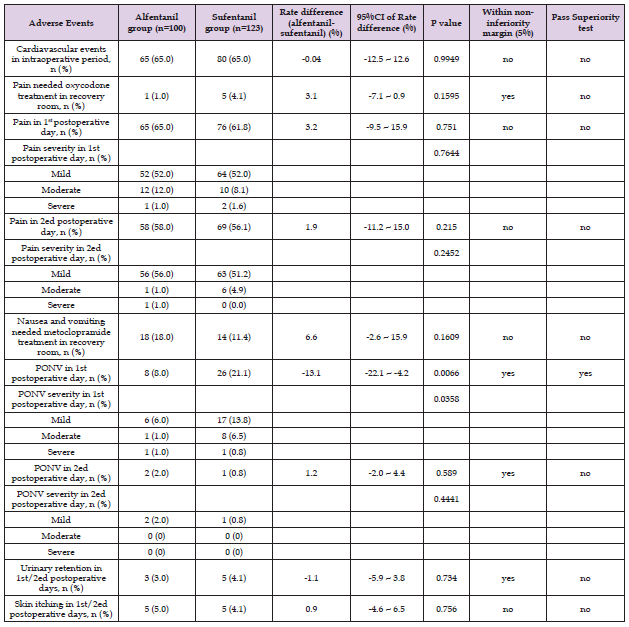
Comparison of Effects of Alfentanil and Sufentanil Groups in the Recovery Room: The incidences of undesired pain that needed oxycodone treatment, and nausea and vomiting that needed metoclopramide treatment were measured and compared between alfentanil group and sufentanil group in the recovery room (refer to Table 4). The undesired pain (VAS ≥ 4/10) requiring oxycodone treatment occurred in 18 out of 100 participants in alfentanil group vs. 14 out of 123 participants in sufentanil group. The incidence was 6.62% higher in alfentanil group than sufentanil group, but did not reach the statistical significance, P = 0.1609. Nausea and vomiting occurred in 1 out of 100 participants in alfentanil group vs. 5 out of 123 participants in sufentanil group. The incidence was 3.07% lower in alfentanil group than sufentanil group, but did not reach the statistical significance, P = 0.1595. These results suggest that alfentanil and sufentanil have the similar effects on pain and nausea and vomiting in the early postoperative period.
Comparison of Adverse Effects of Alfentanil and Sufentanil in the First 2 Postoperative Days: On the first postoperative day, pain occurred in 65 out of 100 participants in the alfentanil group and 76 out of 123 participants in the sufentanil group. The incidence rate was 3.21% higher in the alfentanil group, with the 95% confidence intervel of the rate difference between -9.48 % and 15.91%, P = 0.751. There was no significant difference in the distribution of mild, moderate, and severe pain degree between the two groups, P = 0.7644. Nausea and vomiting occurred in 8 out of 100 participants in alfentanil and 26 out of 123 participants in sufentanil group. The incidence rate was 13.1% lower in alfentanil group than sufentanil group, with the 95% confidence interval of the rate difference between -22.10% and -4.18%, which meets the non-inferiority (margin = 5%) hypothesis and also reachs superior level, P = 0.0066 (refer to Table 4 and Figures 3 & 4). The mild, and moderate nausea and vomiting was significantly less in the alfentanil than in sufentanil group, P = 0.0358 (ref to Table 4 and Figure 4). On the second postoperative day, pain occurred in 58 out of 100 participants in the alfentanil group and 69 out of 123 participants in the sufentanil group. The incidence rate was 1.9% higher in the alfentanil group, with the 95% confidence intervel of the rate difference between -11.45 % and 14.96%, P = 0.215. There was no significant difference in the distribution of mild, moderate, and severe pain degree between the two groups, P = 0.2452. Nausea and vomiting occurred in 2 out of 100 participants in alfentanil and 1 out of 123 participants in sufentanil group. The incidence rate was 1.2% higher in alfentanil group than sufentanil group, with the 95% confidence interval of the rate difference between -1.98% and 4.36%, P = 0.589. There was no significant difference between the two groups regarding the severity distribution, P = 0.4441. On the first two postoperative days, 3 out of 100 participants had urinary retention in the alfentanil group than 5 out of 123 participants in the sufentanil group. There was no significant difference between the two groups, P = 0.734; 5 out of 100 participants occurred skin itching in the alfentanil group than 5 out of 123 participants in the sufentanil group. There was no significant difference between the two groups, P = 0.756.
During the anesthesia induction period, the mean adverse effects of opioids are cough, muscle rigidity, and bradycardia and hypotention. As the primary endpoint of this study, incidence of cough in the induction period was applied to be basis for calculating sample size. Based on the relative potency of alfentanil and sufentanil with meperidine [1], we calculated that the titer ratio of alfentanil to sufentanil was about 1:65. Thus, the experimental drugs in this study were prepared accordingly into equal potency per unit volume, and the consumed volume were compared between the two groups. In the induction period, the medication for each participants followed the same protocol, and the cardiovascular effects in the participants of alfentanil and sufentanil groups were comparable (ref to Table 3). The incidence of muscle rigidity was also comparable in the two groups. However, the incidence of cough was significantly lower in the alfentanil group than in sufentanil group (12% vs. 22%, non-inferior analysis showed positive, further more reached the superior level, P = 0.0412) (ref to Table 2), which proved the priority of alfentanil over sufentanil on cough-inducing side effect. The possible mechanism of opioid induced cough may be its connector μ receptor activation effect, then activation of presynaptic sensory C fibers and release of neuropeptides, leading to sudden contraction of bronchi and vocal cords [7]. Moreover, citrate, a preparation of opioids is also a cough-inducing substance. Whereas China domestic alfentanil hydrochloride is prepared with hydrochloride, which could be one of the reason of its lower incidence of inducing cough than sufentanil citrate. Agarwal et al reported that when alfentanil 10 mcg/ kg was slowly injected for more than 10s, the incidence of cough was 7.2% [8]. Other investigators reported that when alfentanil 8 mcg/ kg was injected within 5s, the incidence of cough was 8% [6]. These reports imply that the dose and speed of alfentanil injection are related to the incidence of cough. Our data are consistent with these reports. The higher incidence of cough induced by alfentanil (12%) may be due to the higher dose we used. The protocol of this study aimed to provide similar anesthesia effects for all the participants. The BIS monitoring was applied to provide a more reliable reference for the depth of general anesthesia, while continuous ramifentanil pumping was applied to maintain adequate analgesia to keep cardiovascular stability. This goal was achieved, for the time to exbutation and the Ramsey score 30 minutes after extubation were comparable between the two groups in the recovery room.
To maintain an appreciate depth of general anesthesia, 1% sevoflurane (about half of its MAC) inhalation was applied throughout the anesthesia, combined with 1% propofol continuous pumping at an adjusted rate to maintain the BIS value between 40~60. Therefore, the consumption of propofol per unit time could be an indicator of hypnotic demand beyond sevoflurane hypnosis of the participants during operation. Ramifentanil characterized by its strong, prompt and extremely short analgesia effect. So, ramifentanil continuous pumping was applied as a supplement for inadequate analgesia during operation. Therefore, the consumption of ramifentanil per unit time represented the deficiency of analgesia effect provided by alfentanil or sufentanil during operation. We compared the consumption of propofol and ramifentanil per unit time and total dose of alfentanil and sufentanil during anesthesia to show the relative hypnotic and analgesic needs of participants in alfentanil and sufentanil groups. The total dosage of alfentanil (or sufentanil) and per umit time dosage of propofol were significantly higher in the alfentanil group than those in sufentanil group (4.9±0.9 vs. 4.6±1.0 ml, P = 0.024; 2.9±1.0 vs. 2.6±0.9 mg/kg/h, P = 0.027, respectively), whereas the per unit time dosage of ramifentanil was comparable in the two groups. These results suggested that the hypnotic and analgesic effects of China domestic alfentanil could be weaker than those of sufentanil when the titer ratio of alfentanil to sufentanil was set to 1:65. However, considering of profile of the shorter action time of alfentanil than sufentanil, the "weak" could be due to the relatively long operation time. This is why alfentanil is usually recommended to be applied in short and small procedure [2]. In other words, it could be a limitation of this study that the procedures involved in the study is relatively longer and/or bigger than expected. In shorter and smaller procedures alfentanil might not be "weaker" than sufentanil, for example, painless gastrointestinal endoscopy, colorectal endoscopy, or fiberoptic bronchoscopy [2-4].
However, we did not detect any difference between alfentanil group and sufentanil group regarding pain assessment in the first 2 days after operation, including incidence and severity of pain (ref to Table 4), at the basis of restricting other analgesics, such as non-steroid anti-inflammation drugs (NSAIDs) application before patients required for pain killer and the VAS scores exceeded 3/10. These results indicate that although the hypnotic and/or analgesic effects of China domestic alfentanil during surgery may not be as good as those of sufentanil, its analgesic effect after surgery is equivalent to that of sufentanil. Célérier, et al. [9] showed that intraoperative alfentanil had no or less pronociceptive effect than sufentanil and remifentanil in mice. Thus we presume that intraoperative alfentanil than sufentanil causes less hyperalgesia after surgery, which may be a explanation to our observation. This issue needs further investigation.
The incidence of PONV was significantly lower in alfentanil group than in sufentanil group (ref to Figure 3). PONV was most obvious in the 1st postoperative day in both groups, 8 out of 100 participants suffered from PONV in the alfentanil group whereas 26 out of 123 in the sufentanil group. The non-inferiority test revealed valid in favor of alfentanil group, and also reached superior level (ref to Table 4). Moreover, most PONV occurred in alfentanil group were mild whereas some moderate PONV occurred in sufentanil group. These results indicate that China domestic alfentanil is superior to sufentanil in term of PONV adverse effect, which coincides to the previous literature reports about alfentanil characteristic of lower PONV incidence in opioids [10,11]. In this study, muscle rigidity might occur after intravenous injection of alfentanil and sufentanil. However, the incidence of muscle rigidity between the two groups is comparable (14.6% vs 14.8%, P=0.9523), and the distribution of the severity of muscle rigidity between the two groups is similar (refer to Table 2, most of the severity is mild, P=0.9216). It was reported that high dose of alfentanil (175 mcg/kg) 100% induced severe muscle rigidity, leading to an immediate increase in central venous pressure (CVP), deoxygenation, and acidosis [12]. Though in this study the muscle rigidity occurred in only nearly 15% of cases, and most of them were not severe, cautions should be exercised in the clinical practice, especially in patients with cardiovascular complications. The reason for the much lower incidence and severity of muscle rigidity after injection of alfentanil could be the much lower dose applied. Though alfentanil has a very high therapeutic index (LD50/ED50 = 1080) [5], over ose than needed could cause undesireable effects.
Incidence of other adverse effects such as skin itching and urine retension in the postoperative period were quite low in this study (ref to Table 4). We did not detect any difference between alfentanil and sufentanil groups regarding these adverse effects. There are other limitations in this study. First, the participants did not included patients of other nations. Because there are very few patients from other countries in the hospital, we do not include these groups to avoid racial bias. Second, the most concerned adverse effect of opioids, respiratory depression was not studied in this study. Literature [13] has shown that the plasma concentration with moderate to strong analgesic effect of alfentanil is lower than the respiratory depression threshold, whereas that of sufentanil and fentanil is higher than their respiratory depression threshold, which implys that alfentanil is less respiratory-depressive than sufentanil and fentanil in clinical practice. But in this study context, it was hard to perform the evaluation of respiratory function. A specific study design is needed for that purpose of experiment. Third, the dosage of postoperative analgesic drugs was not recorded in detail, which may be a confounding factor in the comparing the postoperative analgesic effects between alfentanil group and sufentanil group. Next, the data about incidence of postoperative urinary retention was not reliable, because there were many urological surgeries involved in this study and dwelling catheters were usually applied after operation, which affected the evaluation of drug induced urinary retention.
In conclusion, the data of this clinical study show that the efficency of China domestic alfentanil is similar to that of sufentanil at a potency ratio of 1:65. Alfentanil is superior to the equivalent dose of sufentanil in inducing the adverse effects of opioid-induced cough and PONV in relatively short and small elderly surgeries.
We would like thank Prof Bing-wei Chen, School of Public Health, Southeast University, for his help in the statistical analysis of this study. Yichang Human Well Pharmaceutical Co. Ltd., Yichang, China financially supported this work. They did not involve in the editing of the protocol, drug supply, data analysis, or writing of the paper. Their only requirement was to provide the experiment report at the end of the experiment. The drugs studied, China domestic alfentanil hydrochloride and sufentanil citrate, are among the productions of the sponsor, Yichang Human Well Pharmaceutical Co. Ltd., Yichang, China. Yichang Human Well Pharmaceutical Co. Ltd., Yichang, China signed a contract with Department of Anesthesiology, Zhongda Hospital affiliated to Southeast University to support this study and required the provision of the experiment report at the end of experiment. The copyright of this study belongs to Zhongda Hospital affiliated to Southeast University. There is no other known conflict of interest that could affect this work.
There is no presentation elsewhere declared.


