Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Fahima M Helaly1, Sally A Abou Taleb2, Bassant M Ibrahim3, Reda M Mourad1 and Eman AboBakr Ali1*
Received: January 30, 2023; Published: February 14, 2023
*Corresponding author: Eman AboBakr Ali, Polymers and Pigments Department, National Research Centre, Dokki, Giza, Egypt
DOI: 10.26717/BJSTR.2023.48.007686
This work aims to prepare the promising responsive terpolymer nanoparticles for targeting and sustained release of natural therapeutic Curcumin (Curm) and/or active agents Donepezil hydrochloride (DH). N-isopropyl acrylamide - 2 (dimethylamino) ethyl methacrylate – butyl methacrylate (B5) and N-isopropyl acrylamide - 2 (dimethylamino) ethyl methacrylate – styrene (S5) terpolymers were successfully synthesized and characterized by spectral analysis. FT-IR, 1H-NMR, and scanning electron microscopy (SEM), confirmed the structure of terpolymers as well as their nanoparticle formulas. Encapsulation efficiency of Curm in B5 at two different feed ratios (1:5B5 and 5:1B5) was found to be 96.01 and 89.3%, respectively, whereas for S5, it was 98.01 and 97.1%, respectively. S5 exhibited the smaller drug loaded particle size 14. 13 and 10.24 nm for Curm and DH, respectively. The mean zeta potential of the functionalized polymeric nanoparticles ranged between −13.80±7.34 mV to −7.36±6.63 mV. In vitro study shows a sustained release phase. In vivo investigation revealed that the treatment with Donepezil produced significant improvement in cognition by utilizing either conventional or polymer-loaded high doses Donepezil polymer loaded high dose exhibited the best result in lowering the cholinesterase most probably due to higher ability to pass the blood brain barrier. Meanwhile, the curcumin polymer loaded low and high formulas best results was attributed to its high antioxidant capacity rather than a direct effect on brain.
Over the years, polymeric delivery systems have been devoted to overcoming the disastrous consequences of using specific medications [1,2]. Among all, stimuli-responsive polymeric drug delivery systems have emerged as promising tools for targeting and controlling the release of therapeutic agents [3-5]. In particular, stimuli-sensitive nanoparticles enabled advances in minimizing the dosage frequency while targeting and maintaining drug concentration for long period. Poly(N-isopropylacrylamide) (pNIPAM) is a well-known type of stimuli-sensitive polymer [6,7]. It demonstrates a swollen state with hydrated water molecules at a temperature below the lower critical solution temperature (LCST) of it (32 °C). At higher temperatures, significant contraction (by ~40%) is observed due to breaking down the hydrogen bonding between the polymer and water molecules [8]. This led to water being expelled from the polymeric matrix. Many studies have been reported its copolymers with acidic or basic comonomers to serve in many application [9-12]. For example, Elmas et. al synthsised a temperature sensitive-fluorescent probe used for quantitative determination of diol by copolymerization of NIPAM with 4-vinylphenylboronic acid [13]. Likewise, much attention has been directed to dual responsive of NIPAM copolymers. Copolymerization of two or more monomers containing two or more functional groups allows the copolymer to have more functionalities as an environmentally responsive polymer. Also, It is more flexible to control drug release when multiple stimuli-responsive polymers are present, such as a thermo- and pH-responsive polymer.
From a biomedical point of view, temperature and pH are two important environmental factors in biological and physiological systems. For example, A thermoresponsive cationic nanogel network was prepared based on N-isopropylacrylamide (NIPAM), 2-(dimethylamino)ethyl methacrylate (DMAEMA) and quaternary alkyl ammonium halide salts of DMAEMA to investigate their potential in gene delivery [14]. Since, 2-(dimethylamino) ethyl methacrylate (DMAEMA) is a cationic, water soluble polymer below its pKa value of pH 7.4. DMAEMA demonstrates a cloud point as a function of pH and temperature value. It’s (LCST) in the range 35–45 °C [15]. Another research reported the preparation of thermo sensitive nanoparticles based on NIPAM and DMAEMA. They were tested for encapsulation of hydrophobic anticancer agent [16]. In this work we aimed to synthsize terpolymer based on NIPAM for the development of noninvasive drug delivery strategy for Alzheimer’s disease (AD). Most of the drugs intended to treat this disease facing a challenge to overcome what is called the blood–brain barrier (BBB). The BBB can only be crossed passively by hydrophobic molecules with a molecular weight below 500 Da [17]. Therefore, stimuli-responsive polymeric nano materials are promising candidates to achieve the controlled release of drug at the target sites. Herein, we successfully prepare polymer based on NIPAM with incorporation of DMAEMA to induce polymer hydrophilicity.
Also, smaller ratio of hydrophobic monomers were incorporated for better conjucation of therabutic agent as well as penetration of BBB. N-isopropyl acrylamide - 2 (dimethylamino) ethyl methacrylate – butyl methacrylate (NIPAM-DMAEMA-BMA) and N-isopropyl acrylamide-2 (dimethylamino) ethyl methacrylate – styrene (NIPAM-DMAEMA-St) were synthesized by emulsifier-free emulsion polymerization. Then, they were fabricated in nanoforms and tested for In vitro and In vivo delivering of curcumine and Donepezil hydrochloride (DH) as therabutic agent for Alzheimer’s disease.
Materials
N-isopropyl acrylamide (NIPAM), 2 (dimethylamino) ethyl methacrylate (DMAEMA) butyl methacrylate (BMA), were purchased from. Styrene (St), glutraldhyde, polyethylene-glycol, potassium persulfate (K2S2O8), aluminum chloride (AlCl3) and were products of Sigma-Aldrich. Curcumin (Curm), Donepezil hydrochloride (DH) were products of Acros.
Animals
Female Wistar Albino rats, weighing 180–200 g, were used. The rats were obtained from the animal house colony of the National Research Centre (NRC), Egypt. The animals were kept in standard plastic cages in an air-conditioned room at 22±3 °C. The humidity was 55±5 % and they were supplied with standard laboratory diet and water ad libitum. All experimental procedures were conducted in accordance with the guide for the care and use of laboratory animals. Experimental procedures and use of laboratory animals followed the recommendations of the National Institutes of Health (Publication No. 85-23, revised 1985).
Kits
Kits for detection of cholinesterase purchashed from Elabscience, USA.
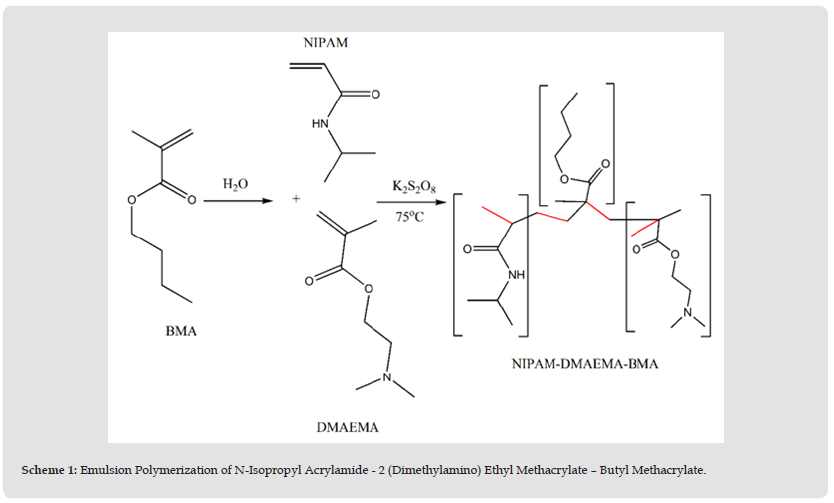
Emulsion Polymerization of N-Isopropyl Acrylamide-2 (Dimethylamino) Ethyl Methacrylate – Butyl Methacrylate
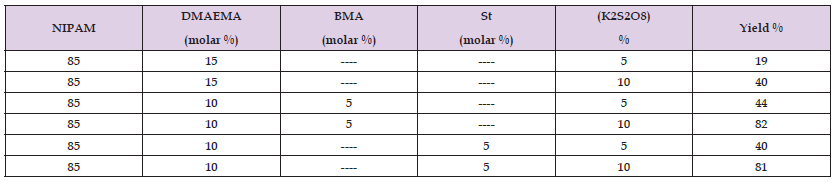
A certain amount of butyl methacrylate was mixed vigorously by highspeed homogenizer with a known volume of distilled water. After three minutes, N-isopropyl acrylamide-2 (dimethylamino) ethyl methacrylate were mixed to the emulsion. The ratio of monomers is depicted in (Table 1). K2S2O8 was added to the mixture then, it was bubbled with nitrogen for 15 min. The polymerization was carried out by raising the temperature to 75 oC for 4 hours.
Emulsion Polymerization of N-Isopropyl Acrylamide-2 (Dimethylamino) Ethyl Methacrylate – Styrene
A certain molar rotio of styrene, as illustrated in (Table 1) was mixed vigorously by high speed homogenizer with a known volume of distilled water. The same condition and procedure presented in section 1.2 were implemented for this copolymerization.
Fabrication of the Functionalized Biodegradable Polymeric Nanoparticle Delivery Systems
Preparation of the Functionalized Biodegradable Polymeric Nanoparticle Formula: The functionalized biodegradable polymeric nanoparticles were prepared by dissolving the synthesized functionalized polymer in a 10 mL ethanolic aqueous solution. Glutraldhyde and polyethylene-glycol were added to the mixture in ratios 1:5 and 5:1. After connecting to a cooling condenser, the mixture sonicated for 3hrs. Then, in a cold water-bath shaker, the mixture was left overnight. After that, it was centrifugated, then washed several times. Finally, dried by freez-drying.
Loading the Drugs in the Functionalized Biodegradable Polymeric Nanoparticle Formula: Curcumin and Donepenzil HCl were physically entrapped in the hydrophobic core of the polymeric nanoparticle micelles using vortexing and sonication methode. Briefly, the lyophilized powder of the synthesized polymeric nanoparticle was dispersed in a 2mL of water and ethanolic solution, where (2 mg/ mL) of the drugs were separately and gradually added. The mixture was vortexed and sonicated (1 min, 35% amplitude) till a clear nanoparticle suspension occured. Upon lyophilisation, fine powder.of drug-loaded polymeric nanoparticles was achieved.
Process Yield Determination of the Functionalized Biodegradable Polymeric Nanoparticle Formula: The process yield (PY) was determined as following [18].

Evaluation of Drug Loading Capacity and Encapsulation Efficiency: The drugs encapsulation efficiency (EE) percentages of the prepared nanoparticles were determined by the following method: In brief, certain amount of drug loaded nanoparticles (10 mg) were taken into 5ml alcoholic buffer solution, and the nanoparticles suspensions were allowed for centrifugation at 10,000 rpm for 30 min. The drug content in the supernatants was analyzed by UV-spectrophotometer. Each batch sample was measured in triplicate.
The EE percentages were calculated by the following Equations:
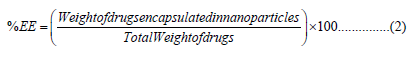
In Vitro Release Study: The In Vitro release studies were carried out as follows: drugs loaded nanoparticles and a 2ml Phosphate-buffered saline(PBS) solution (pH 7.4) were placed into a dialysis tube (M.W-CO: 15,000). These dialysis tubes were put individually in 100 ml PBS at 37 ◦C and maintained under shaken at 100 rpm. Triplicate analysis for each sample was performed. At specific time intervals, 5ml media was taken and replaced with equivalent amounts of fresh PBS. The concentration of the released drugs into PBS were determined by UV spectrophotometer.
Release Kinetics: Data obtained from the in vitro release studies of drugs loaded nanoparticles were fitted to various kinetic equations such as zero, first, second, third orders, Higuchi model and Korsmeyer–Peppas model [19].
Characterization
Fourier-Transform Infrared Spectroscopy (FT-IR): FTIR spectra were measured at a resolution of 4 cm-1. The range of measurement was 400 to 4000 cm-1 using spectrometer (Nicolet, 5DX/550II, USA).
Proton Nuclear Magnetic Resonance (H-NMR): 1H-NMR of the samples were recorded using a JEOL-ECA-50 NMR instrument 500 MHz. DEMSO was taken as chemical shift reference.
Scanning Probe Microscope: Samples particle size were scanned at various scan areas using Shimadzu SPM 9500-2J Scanning Probe Microscope. For high resolution, a contact mode cantilever was used for all analyses.
Dynamic Light Scattering (DLS): Zeta potential of nanoparticles were separately measured in deionized distilled water [20] using Zeta-sizer 3000 HSA (Malvern Instruments, U.K.).
Scanning Electron Microscopy (SEM): The surface morphology of the prepared copolymers and nanoparticles were identified by a LEO Electron Microscopy (Inc., Thornwood, NY) operating at 3 kV with a filament current of about 0.5 mA.
In vivo Pharmacological and Biological Evaluation of Alzahiemer Disorder (AD)
The donepezil dosage was chosen on the basis of information from previous in vivo studies: Donepezil Polymer loaded nano formula 0.0075 mg/kg, Curcumin 250 mg/kg and Curcumin Polymer loaded nano formula 2.5 mg/kg.
Study Design
Eighty adult female Wistar Albino rats were divided into 8 groups (ten each). The first group (negative control group), rats were given a daily oral dose of 2 mL distilled water throughout the experiment. Second group (positive control) in which a daily oral administration of AlCl3 were given to rats in a dose of 100 mg/kg for three successive weeks to mimick AD [21]. Third group (treated rats) includeed orally given donepezil hydrochloride (0.75 mg/kg) used as a standard drug [21] , donepezil nano formulas in high and low doses as well as conventional curcumin (250 mg/kg) and curcumin loaded nano formulas in high and low doses respectively, for three weeks in combination with AlCl3 (100 mg/kg).
Biochemistry Study
Blood Sampling: At the end of the experiment, 18 h food-deprived rats blood samples were withdrawn from the retro-orbital venous plexus. The collected blood samples were kept to stand for 10 min at room temperature. Then they were centrifuged at 4oC using cooling centrifuge (Laborezentrifugen, 2k15, Sigma, Germany) at 3000 r. p. m for 10 min. After that, sera were separated for the assessment of serum level of cholinesterase according to the methods of Satoh,1978, Beutler et al,1963.
Biochemical Parameter: Cholinesterase:
Principle: The Sandwich-ELISA principle was applied to test the samples by using enzyme linked immunosorbent assay (ELISA) kit. The provided micro ELISA plate in this kit has been pre-coated with an antibody specific to Rat AChE. Samples were added to the micro ELISA plate wells to be combined with the specific antibody. Then, a biotinylated detection antibody specific for Rat AChE and Avidin- Horseradish Peroxidase (HRP) conjugate were added consecutively to each micro plate well and incubated. Free components were washed away. The substrate solution was added to each well. The blue color appeared indicating biotinylated detection antibody and Avidin-HRP conjugate for those wells that contain Rat AChE. Then, by adding of stop solution, the terminationof the enzymesubstrate reaction were carried out and the color turns yellow. The optical density (OD) was measured spectrophotometrically at a wavelength of 450 nm ± 2 nm. The OD value is proportional to the concentration of Rat AChE. By comparing the OD of the samples to the standard curve, the concentration of Rat AChE in the samples was calculated. The test procedure followed manufacturers guidelines (Catalog No: E-EL-R0355 Product size: 96T/48T/24T/96T*5 Rat AChE(Acetylcholinesterase) .
The emulsifier-free emulsion polymerizations technique was utilized to prepare thermosensitive and pH sensitive terpolymers. In order to initiate polymerization, the mixture solution with the monomer and K2S2O8 was heated to 75 °C. Generally, the thermal initiator, dissociates and produces free radicals which interact with monomers to form active site to proceed with the polymerization process. Different ratio of BMA and St with DMAEMA to a fixed amount ofNIPAM (Table 1) were studied to reach the optimum concentration that suits the following nanofabrication procedure. The introduction of NIPAM to have LCST of 32 °C in the polymer matrix will lead to thermoresponsive behaviour, whereas the varied molar ratio of DMAEMA enhance the pH responsivity. Initially, BMA or St were dispersed vigrously in water with high speed homogenizer to form smaller droplets that will be easily diffuse in the other mnomer solution through polymerization process. Styrene’s solubility in water has been reported to be 0.03% at 20 °C, and it may increase to 0.96g% at 60 °C [22]. This behavior most probably will lead to fast interaction between NIPAM and St in the emulsion polymerization. DMAEMA has many edges in this polymerization process. Besides adding the hydrophylic and pH sensitve characteristic, DMAEMA enhances the polymerization rate. As the polymerization proceeds, methacrylates derivatives are usually more reactive than acrylamide ones in the binary copolymerization [23].
Also, bearing in mind the high reactivity ratio of BMA towards DMAEMA as previously reported (r1=1.26, r2=0.97, respectively) [24], NIPAM is totally consumed in a rapid polymerization. Our goal was to prepare water-soluble terpolymer so for further work we chose B5 and S5.
Emulsion Polymerization of N-Isopropyl Acrylamide-2 (Dimethylamino) Ethyl Methacrylate–Butyl Methacrylate
According to the experimental part, (Scheme 1) shows the emulsion polymerization condition. The effect of initiator concentration was studied. (Table 2) shows the monomer concentration, initiator concentration, and the yield percent.
Figure 1 Scheme 1: Emulsion Polymerization of N-Isopropyl Acrylamide - 2 (Dimethylamino) Ethyl Methacrylate – Butyl Methacrylate.
Emulsion Polymerization of N-Isopropyl Acrylamide - 2 (Dimethylamino) Ethyl Methacrylate – Styrene
Styrene was incorporated in the emulsion polymerization with a similar molar ratio of butyl methacrylate. (Table 2) illustrates the yield percent of the prepared copolymer. (Scheme 2) represents the proposed condition of emulsion polymerization.
Table 2: Composition of Emulsion Polymerization of NIPAM – DMAEMA with BMA or St in Distilled Water at 75oC.
FTIR Analysis: (s 1) shows FT-IR spectra of the NIPAM-DMAEMABMA and NIPAM-DMAEMA-St terpolymers. The broad absorption peaks observed in the range of 3000– 3600 cm-1 are assigned to the stretching N-H groups in NIPAM. The observed peaks in the domain 2800-3000 cm-1 are due to the asymmetric and symmetric stretching of C–H bonds in the methylene groups. The absorption peaks at 1622.62 – 1659.73 cm-1 belonged to the amide peaks of NIPAM units. The peaks at 1385 and 1456 cm-1 are attributed to -CH3 groups in DMAEMA moieties. The well-defined band at 1730 cm-1 related to the vibrational stretching of ester carbonyl. Other bands at 2772 and 2836 cm-1 are due to stretching C–H groups in N(CH3)2 [25]. The peak at 1154 cm-1 is related to the C–N elongation of tertiary amine. At region 2963–2879.5 cm-1, the peaks stand for the CH stretching frequencies of CH2 of BMA copolymer (Figure 1a), while 897.66- 724.56 cm-1 peaks represent the bending frequency of aromatic CH of St copolymer as shown in (Figure 1b).
1H-NMR Analysis: As shown in Figure 2, 1.25- 1.4 ppm signals are assigned to–CH2 and CH3 adjacent to BMA and NIPAM. The methylyne group attached to the carbonyl group signal is detected at δ 4.4 -4.7 ppm. In (Figure 2) and (Figure 3), the signal at δ 8.0 ppm indicates the presence of amino group of NIPAM. The signal at 3.9 stands for CH group attached to NH group of NIPAM. The confirmation of the participating of St was observed by the signal at 7.0- 7.4 ppm (Figure 3).
Surface Morphology: Using Scanning electron microscopy (SEM), surface of B5 and S5 has been studied (Figure 4). A homogenouse surface with pattern of physical crosslinking is observed for B5 sample. Also, S5 image shows a homogenous matrix but with large cohesion compared with B5.
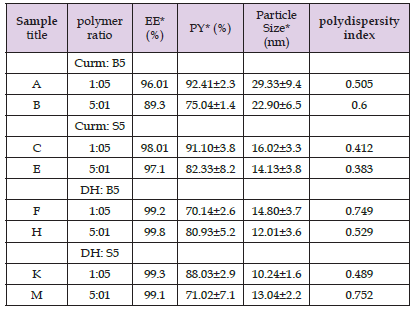
Table 3: Process Yield, Encapsulation Efficiency, Particle Size, and Polydispersity Index of Nanoparticle Formulations.
Note: *(n=3±S.D.)
Process Yield, Encapsulation Efficiency, Particle Size, and Polydispersity Index Data of Nanoparticle Formulations
The nanoparticles were successfully prepared, showing clearer nano-formulations for those systems that were fabricated with higher polymer ratios. The process yield, particle size and polydispersity index of nanoparticles are given in (Table 3) The higher process yield was ranging between 85.93±5.2% and 92.41±2.3% depending on the polymer ratio used. The size of drug loaded nanoparticles was between 8.36±2.2 nm and 29.33±9.4 nm. The mean zeta potential of the functionalized polymeric nanoparticles ranged between −13.80±7.34 mV to −7.36±6.63 mV indicating the formation of stable, uniform nanoparticle systems. The polydispersity indexes of all formulations were beneath the one reflecting the proper homogeneity of the fabricated nano-systems. Depending on the previous results of drugs loaded nanoparticles, sample C, E, K and H revealed the most reasonable data and thus selected for further studying.
FT-IR Analysis of Nanoparticle Formulations
The diminutive effect on characteristics peaks or presence/ absence of such peaks associated with the specific structural groups of each active molecule used in the preparation of drugs loaded nanoparticles was used to prove the formation of nanoparticle and the interaction of drugs with the formatted nanoparticles. The chemical interaction was reflected by changes in the characteristics peaks of the actives depending upon the degree of interaction. In general, minor peaks (lesser intensity) or peak shifts were observed (Figure 5) in the drugs loaded nanoparticles spectra when compared with the pure drugs and the polymers spectra. Drugs loaded nanoparticles SEM photos (Figure 6) showed a spongy-like network structure, and it was observed that all of them revealed polydisperse, and porous semi spherical shapes. Particles encapsulating drugs were observed to be large spheres with visible pores enclosing drug particles inside.
In Vitro Release Study and In Vitro Release Kinetics
The In Vitro cumulative release profiles of drugs loaded nanoparticles in the phosphate buffer solution (PBS)(pH 7.4) are shown in (Figure 7). There was an initial burst release phase and a sustained release phase in the release profiles. About 11% of drugs were released in the burst release phase (within the first release hour), which was mainly because the nanoparticles surface drugs could easily diffuse in the first phase. And the second phase was a relatively slow release up to 24 h, which might be attributed to the drug diffusion and the polymer degradation. The drugs- loaded nanoparticles with low polymer ratios revealed a faster release than their correspondence nanoparticles with high polymer ratios, thus nanoparticles with high polymer ratios exhibited stronger sustained release effects. The release data interpretation of the drugs loaded nanoparticles showed that maximum linearity (highest R2 value) was found with Korsmeyer–Peppas model, thus substantially fit into such model, which was used to determine the mechanism of drug release at the initial portion (i.e., Mt/M∞ ≤60%), and it’s equation is as the following:
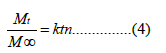
In the above equation, n represents the diffusion exponent and k is a parameter. The values of n and correlation coefficient (R2) have been calculated and the obtained results are shown in (Table 4). The values of n deviated from 0.50, indicated that the drug’s release followed non-Fickian or anomalous diffusion. It may result from the degradation of the polymer and the rate of fluid ingress into the matrix [26].
In vivo Pharmacological and Biological Evaluation of Alzahiemer Disorder (AD)
Results of Cholinesterase in Serum (Figure 8), shows a nonsignificant difference in its level in groups treated with Donepezil (0.75mg/kg), curcumin (250 mg/kg) and Donepezil (0.0075 mg/ kg) polymer loaded high dose when compared to the normal group. Meanwhile the positive control group, Donepezil polymer loaded low dose as well as Curcumin polymer loaded low and high doses showed a significant increase in levels of cholinesterase in serum when compared to the normal group. Results are expressed as means of % of serum level of cholinesterase in serum ± SE, n=10. significance at p ˂ 0.05*. Significantly different from normal control group.@ Significantly different from positive control group. &Significantly different from Curcumin 250 mg/kg group. Comparison was done using One way analysis of variance followed by Tukey Kramer test using Graph Prism software version 8.
Using the emulsion approach, a new class of polymeric nanocarriers was developed. Spectral characterization confirmed the polymer’s structure. The nanoparticles were successfully prepared with higher polymer ratios. NIPAM-DMAEMA- BMA and NIPAMDMAEMA- St terpolymers stand efficient for the encapsulation of hydrophobic (Curcumin) and hydrophilic compounds (Donepezil HCl). However, NIPAM-DMAEMA- St shows a smaller nanoparticle size compared with NIPAM-DMAEMA- BMA. Donepezil in conventional form and in high polymer concentration loaded form acted through both cholinesterase inhibitory effect as both forms significantly reduced cholinesterase together. The investigated polmers improved the release of both curcumin and Donepezil. The effect is concentration dependant in the case of Donepezil only. This might lead to the introduction into the market of a new form of Donepezil which is in lower concentration in the polymer form to minimize its side effects.
The author gratefully acknowledge the finnatial support and the provided facilites by the National Resesrch Centre, Egypt.


