Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Samuel Abiodun Kehinde1,2, Abosede Temitope Olajide2*, Ayokanmi Ore2 and Sanmi Tunde Ogunsanya3
Received: January 23, 2023; Published: February 13, 2023
*Corresponding author: Abosede Temitope Olajide, Department of Chemical Sciences, Faculty of Natural Science, Ajayi Crowther University, Oyo, Oyo State, Nigeria
DOI: 10.26717/BJSTR.2023.48.007679
New research indicates that disruption of renal bioenergetics during acute kidney injury (AKI) development is a notable pathophysiologic event, and bioenergetic failure caused by pyruvate depletion is a key mechanism underlying AKI. The aim of this study is to investigate the effect of DiNP on renal energy transduction. Eighteen rats were divided into three groups of six rats each. Tween-50 was given orally to Group A (control) for 14 days, DiNP (20 mg/kg/BW) to Group B, and DiNP 200 mg/kg to Group C. The rats were sacrificed, and their kidneys were excised. After that, renal enzyme activities for glycolysis, tricyclic acid (TCA), and the electron transport chain (ETC) were determined. Activities of hexokinase and phosphofructokinase-1 were significantly increased relative to the control (p<0.05). Aldolase (ALD), Lactate dehydrogenase (LDH) and NADase activities were down-regulated at 20mg/kg DiNP but significantly increased at 200mg/kg DiNP when compared with the control. In addition, the activity of TCA cycle enzymes and all respiratory chain complexes (Complex I-IV) dropped significantly relative to control. Histopathological examination of the kidney showed deleterious changes in the renal structures of the DiNP-treated rats relative to the control. DINP exposure impairs renal energy transduction enzymes as evident in its downregulatory potential on enzymes of oxidative phosphorylation.
Keywords: Diisononyl Phthalates; Oxidative Phosphorylation; Renal Impairment; Kidney Injury; Mitochondria; Pyruvate Depletion
Abbreviations: AKI: Acute kidney Injury; LDH: Lactate Dehydrogenase; DEHP: Di(2-ethylhexyl) Phthalate; NADPH: Nicotinamide Adenine Dinucleotide Phosphate; CKD: Chronic Kidney Disease
The kidney is one of the organs in the body with the highest metabolic activity. The kidneys use the majority of their energy to maintain fluid and electrolyte homeostasis and prevent vital nutrients from being lost in the urine while removing waste from the body’s metabolism as a whole. According to research, disruption of renal bioenergetics during acute kidney injury is a significant pathophysiologic event. Net gluconeogenesis converts lactate to glucose in the proximal tubule, which is then transported to more distal regions with high glycolytic capacity. One of the main mechanisms underlying acute kidney injury, according to experimental evidence, is poor sodium reabsorption, mitochondrial stress, and pyruvate depletion all contribute to bioenergetic failure [1]. Ninety-five percent of renal ATP is produced in the mitochondria primarily through oxidative phosphorylation. The main substrates for this ATP production include amino acids, glucose, fatty acids, and ketone bodies. The preferred substrate, however, differs along the nephron. For instance, the proximal tubule has a low capacity to metabolize glucose and primarily uses fatty acids, ketone bodies, and amino acids as energy sources, despite the fact that more distal parts of the nephron have a high glycolytic enzyme activity. Contrarily, only in the proximal tubule of the nephron does net gluconeogenesis occur, primarily using lactate as substrate. Such glucose production makes a significant contribution to the body’s overall gluconeogenesis and may also serve as an important energy substrate for the nephron’s more distal portions [1].
DiNP is a relatively recent phthalate ester (PAE) that is utilized as a plasticizer in numerous industrial and consumer plastic goods [2]. DiNP has lately surpassed di(2-ethylhexyl) phthalate (DEHP) and dibutyl phthalate (DBP) as the major plasticizers in polyvinyl chloride (PVC) applications around the world [3]. DiNP goods that are relevant to our everyday lives include hair care products (hair sprays, mousses, and gels), deodorant (including antiperspirants), nail polish, lotions (body lotions and body creams), skin cleansers, and baby products (oils, lotions, shampoos, and diaper creams) [4]. DiNP can be released into the environment via elution, migration, and evaporation due to its strong lipophilicity and non-covalent connection with the plastic matrix just like other PAEs. Human exposure may result from either direct skin contact, use, or environmental contamination [5,6]. DiNP has been demonstrated to interfere with energy metabolism in the heart and testes [7,8]. In rodents, phthalates, including DiNP, have been shown to alter lipid metabolism, specifically fatty acid synthesis and metabolism in multiple tissues during lactation [9,10]. DiNP was discovered to be present in the kidneys of mice in a recent study, and a daily dose of 20 mg/kg body weight/day could cause oxidative damage [11]. One of the explanations for the increased oxidative stress in AKI and CKD is thought to be mitochondrial malfunction and increased mitochondrial ROS.
The main sources of ROS generation are said to include mitochondria, nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase, Xanthine Oxidase (XO), Myeloperoxidase (MPO), and eNOS [12]. Chronic kidney disease (CKD) is a global public health issue due to the rising prevalence and incidence of acute renal injury, kidney failure, poor prognosis (more than 1 million individuals die from untreated kidney failure each year), and expensive expenses [13]. The current investigation sought to look into the potential energy metabolic disruptions caused by DiNP in the kidney using glycolytic, tricarboxylic acid, and electron transport chain enzymes as markers.
Materials
Eighteen Wistar rats weighing 200-230g were purchased from the animal house at the University of Ibadan’s College of Medicine in Ibadan. The rats were placed in plastic cages at Ajayi Crowther University’s animal house in Oyo for acclimation and treatment. The rats were given a week to adapt and were allowed unlimited access to water and pelletized food. Animals were assigned into three groups of six rats each. Tween-80 or 20 or 200 mg/kg BW DiNP (Relonchem Ltd, Cheshire, UK) was administered orally to rats in each experimental group for 14 days. Previous research of [7] and [11] led to the selection of 20 and 200 mg/kg/day DiNP (1:1 of Tween-80 to DiNP was used). All administration was done orally. The study protocol used in this research was approved by the Animal use and Ethics Committee of the Faculty of Natural Sciences, Ajayi Crowther University, Oyo, Nigeria (FNS/ERC/2021/006).
Methods
a. Sample Collection
The rats were cared for and handled according to the published protocols for the care and use of laboratory animals [14]. Under diethyl ether anesthesia, animals were sacrificed and kidneys were removed. The excised kidneys were washed in ice-cold 1.15%, KCl, blotted, weighed, and homogenized with a potter-elevehjem homogenizer in 10 volumes/weight of 0.1 M phosphate buffer (pH 7.4). The homogenate was centrifuged at 10,000 g for 15 minutes at 4oC to collect the supernatant for the biochemical tests.
b. Isolation of Mitochondrial Fraction
As previously described, mitochondria were processed and extracted from the kidney of male Wistar rats [15]. The kidney was excised from the animal, the connective tissues were removed, and the kidney was washed in homogenization medium. The organ was then cut into smaller pieces and rewashed in the medium to remove the blood. It was then dispensed into a sterile 10ml tube. Homogenization medium was poured into the tube at a 5ml/gram of kidney ratio. The organ was then homogenized and centrifuged at 3000rpm for 5 minutes. The supernatant was transferred to a 5ml Eppendorf tube and centrifuged at 13000rpm for 2 minutes. The mitochondria were found in the sediment, which was washed with homogenization medium and centrifuged for 2 minutes at 13000 rpm. The supernatant was decanted and rewashed with MAITE medium. It was centrifuged for 2 minutes at 13000rpm, and the supernatant was decanted. The mitochondria isolate was re-suspended in 1ml of MAITE.
c. Assay of Glycolytic Enzymes
For determination of hexokinase activity, method described by [16] was used. To determine PFK activity, published protocols of [17] were used. The method of [18] was used to measure the aldolase activity. Following the manufacturer’s instructions, the LDH (CYPRESS® Diagnostics, Langdrop, Belgium) activity was assayed following the instruction on as mentioned in the kit manual. Method described by [19] was used to determine the activity of NADase.
d. Assay of Mitochondrial Metabolizing Enzymes
The spectrophotometric enzyme assay reported by [20] was used to assess citrate synthase activity. As previously mentioned, Isocitrate dehydrogenase activity was determined by measuring the reduction in NAD+ at 340nm [21]. The activity succinate dehydrogenase was measured using established protocol [22]. Malate dehydrogenase (MDH) activity was assessed using the specified approach [23]. Complex I (NADH ubiquinone oxidoreductase), Complex II (succinate ubiquinone oxidoreductase), Complex III (cytochrome c oxidoreductase), and Complex IV (Cytochrome C Oxidase) activity in isolated mitochondria were evaluated spectrophotometrically [24].
e. Histopathological Examination
Sections of the kidney tissues from various animals in each group were collected immediately after sacrifice. The tissues were cleansed in normal saline solution (to remove blood), fixed for at least 24 hours in Bouin’s solutions, dehydrated in various grades of alcohol, and prepared for paraffin embedding. Sections were cut using a rotary microtome. The sections underwent treatment and a graded alcohol series before being cleaned in xylene, stained with hematoxylin and eosin (H&E). For photomicrography, the kidney slides were examined under a Leica DM750 camera microscope using objective fields 10 (Mag. x100) for general overview of the histoarchitecture of the kidney and 40 (Mag.x400) for precise identification of the glomerulus, podocytes and adjacent convulated tubules. Photomicrographs of the stained (H&E) sections were subjected to ImageJ software analysis for evaluation and quantification of the histopathological scoring and also determine the severity of the damage in the kidney tissue.
f. Statistical Analysis
The results are given as mean ± SEM. The degree of homogeneity among the groups was determined using analysis of variance (ANOVA).
Tukey’s test was used to distinguish groups with heterogeneity. For all analyses, GraphPad Prism® version 8 was utilized. P values less than 0.05 were considered statistically significant.
Results
a. Effect of DiNP on Renal Glycolytic Enzyme Activities in Rats
As shown in (Figure 1), the activity of renal glycolytic enzymes was significantly altered after DiNP exposure compared to control (A-E). DiNP at doses of 20 and 200 mg/kg body weight upregulated renal hexokinase (p<0.0001; p<0.0153) and phosphofructokinase (p<0.0001; p<0.0001) activity when compared to the control. In addition, at 20mg/kg, aldolase (ALD) (p<0.0001) and lactate dehydrogenase (LDH) activities (p<0.0001) were down-regulated relative to the control group while at 200mg/kg ALD (p<0.0001) and LDH (p<0.0001) activities was upregulated. Finally, when compared to control, NADase activity was markedly decreased, and this reduction was induced by 20 and 200mg/kg DiNP (p=<0.0039; 0.0106).
Figure 1 Effect of DiNP on the activities of renal glycolytic enzymes in rats. Each group’s values are presented as the Mean ±SEM of six rats.
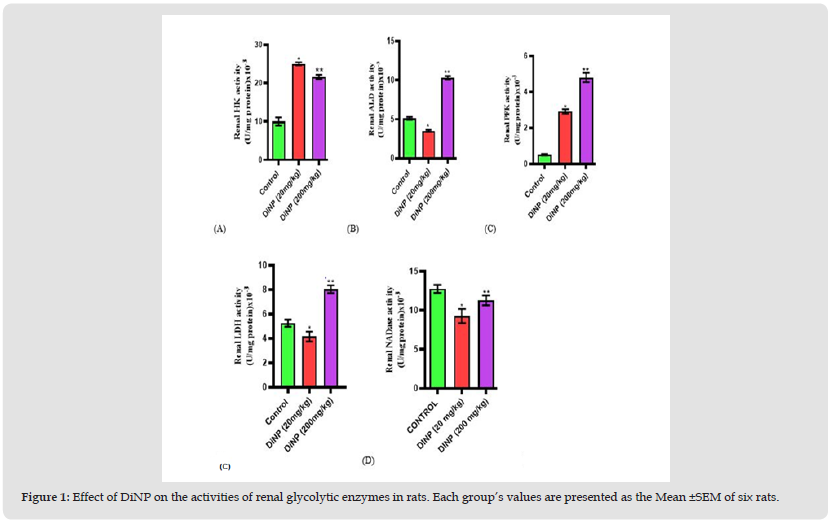
Note: Tukey’s test was performed to test the significant difference between the two groups *= significant difference from the control (p<0.0001; p<0.0153; p<0.0001; p<0.0001). ** = Significant difference (p<0.0001; p<0.0001; (p=<0.0039; 0.0106) from the group receiving DiNP (20 mg/kg). HK= Hexokinase, ALD= Aldolase, LDH= Lactate dehydrogenase, PFK= Phosphofructokinase, NADase= NAD Glycohydrolase.
b. Effect of DiNP on Renal Tricarboxylic Acid Cycle Enzyme Activities in Rats
(Figure 2) depicts the effect of DiNP on the activities of renal tricarboxylic acid cycle enzymes in animals (A-D). Renal citrate synthase activity was significantly (p<0.0001) lower in the animals subjected to the two doses (20 and 200mg/kg) under study than in the control animals. The activity of renal isocitrate dehydrogenase was significantly reduced at 200mg/kg when compared to the control (p<0.0001) while there was no significant difference (p<0.8695) observed at 20mg/kg DiNP, malate dehydrogenase (p<0.0191), and succinate dehydrogenase (p<0.0001) were all markedly decreased relative to the control.
Figure 2 Effect of DiNP on the activities of renal TCA enzymes in rats. Each group’s values are presented as the Mean ±SEM of six rats.
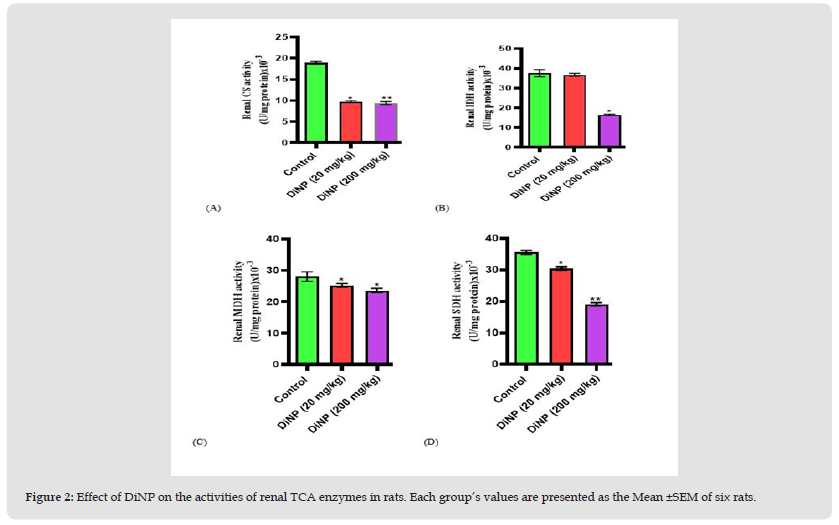
Note: Tukey’s test was performed to test the significant difference between the two groups *= significant difference from the control (p<0.0001; p<0.0001). ** = Significant difference (p<0.0191; p<0.0001) from the group receiving DiNP (20 mg/kg). CS= Citrate synthase, IDH= Isocitrate dehydrogenase, MDH= Malate dehydrogenase, SDH= Succinate dehydrogenase.
c. Effect of DiNP on Renal Electron Transport Chain Enzyme Activities in Rats
The activities of the renal electron transport chain enzymes under investigation were significantly reduced in rats exposed to DiNP compared to controls, as shown in (Figure 3). When compared to the control, DiNP at doses of 20 and 200mg/kg inhibited the activities of renal complexes I (p<0.0001; p<0.0001), II (p<0.0001; p<0.0001), III (p<0.0038; p<0.0001), and IV (p<0.0043; p<0.0001).
Figure 3 Effect of DiNP on the activities of renal electron transport chain enzymes in rats. Each group’s values are presented as the Mean ±SEM of six rats.
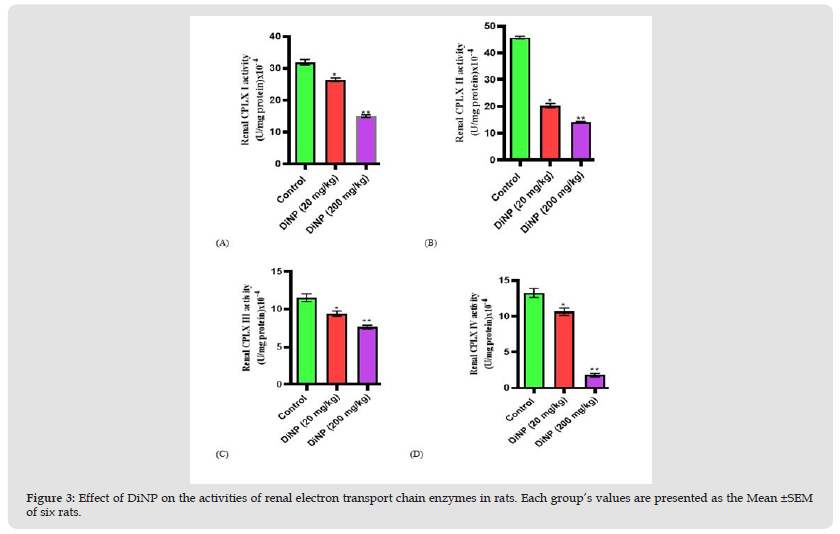
Note: Tukey’s test was performed to test the significant difference between the two groups *= significant difference from the control (p<0.0001; p<0.0001; p<0.0038; p<0.0043). ** = Significant difference (p<0.0001; p<0.0001; p<0.0001; p<0.0001) from the group receiving DiNP (20 mg/kg). CPLX I= Complex I, CPLX II= Complex II, CPLX III= Complex III, CPLX IV= Complex IV.
Figure 4 Representative light micrographs of sections of kidney subjected to H&E stain. Black arrow – Glomerulus, Green arrow – Proximal convoluted tubules (PCT), Blue arrow- Podocyte, Red Arrow – Bowman’s space, yellow arrow – Distal convoluted tubules (DCT), Orange arrow- Bowman’s capsule. A=control; B= 20mg/kg BW; C=200mg/kg BW. White bar: 50 μm.
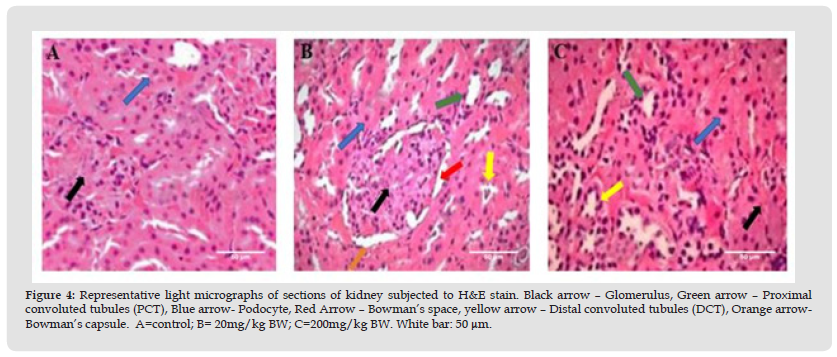
d. Effect of DiNP on Renal Histoarchitecture in Rats
From (Figure 4), histopathological examination of the kidney showed that the renal pelvis and glomeruli were arranged normally in the histological structures of the control group. The mesangium was mostly intact, and the tubular nuclei were visible and normal, with clear outlines of the glomeruli and Bowman’s capsular space and no detectable pathology. The 20mg/kg BW group had mild and temporary hyperemia, hyaline tubule occlusion, and necrosis. The glomerular tissue congestion seen in the histological changes associated with 200mg/kg BW is indicative of necrotic changes. The nuclei tubules were deeply stained and dispersed throughout the mesangial tissues. There is hyaline degeneration of the renal tubules and generalized atrophy of the glomeruli with fibrillary meshwork.
Recent research suggests that disruption of renal bioenergetics plays a significant pathophysiologic role in the onset of acute kidney injury (AKI), and that pyruvate depletion is a critical mechanism causing bioenergetic failure during the development of Acute Kidney Injury (AKI). A variety of substances, including phthalates, can harm the kidneys. The kidney generates ATP through oxidative phosphorylation, and the substrate it uses (amino acids, glucose, fatty acids, and ketone bodies) varies along the nephron [25]. The kidney generates ATP during aerobic respiration by converting glucose to pyruvate, oxidizing acetyl coenzyme A (CoA) from amino acids, pyruvate (decarboxylation catalyzed by pyruvate dehydrogenase), fatty acids, and ketones, and electron transport phosphorylation. Under anaerobic conditions, lactate dehydrogenase (LDH) converts pyruvate to lactate [1]. It has been shown that ATP levels in the renal cortex decrease during AKI while medullary levels remain constant. This finding is consistent with the medullary nephron’s ability to maintain ATP synthesis via anaerobic glycolysis in the face of renal ischemia [26]. Significant changes in metabolic processes occur during AKI, which may have an implication on ATP synthesis. Short-term ischemia (and other types of AKI) stimulates pyruvateinduced gluconeogenesis in the proximal tubule, resulting in glucose as a substrate for distal nephron anaerobic ATP synthesis [27]. It’s significant because increased gluconeogenesis causes pyruvate depletion in this nephron segment, which could lead to AKI pathogenesis.
The findings of this study, which provide the first understanding of how DiNP exposure affects the kidney’s energy metabolism, show that DiNP exposure is linked to changes in the cytosolic and mitochondrial activity of various renal energy-transducing enzymes, which in turn affect cellular energy metabolism. The bonds of the sugar, or glucose, being consumed provide nearly all of the energy required during glycolysis. Several enzymes are used to break down glucose into two molecules of pyruvate. A small amount of energy is released during this process [28]. The enzyme hexokinase aids in the phosphorylation of glucose, which is the first step in the glycolytic pathway. The enzyme hexokinase activity was significantly increased in a dose-dependent manner after DiNP exposure at the two doses used (20 and 200mg/kg) which points to the fact that during exposure to DiNP, glucose is committed for glycolysis in the kidney. Aldolase (ALD) catalyses the conversion of fructose 1-6-diphosphate to glyceralglyceraldehyde 3-phosphate and dihydroxy-acetone phosphate, while phosphofrucokinase-1 (PFK-1) catalyses the conversion of fructose 6-phosphate and ATP to fructose 1,6-bisphosphate and ADP, a «committed» step in glycolysis [29]. It was observed from our findings that DiNP exposure at both doses increased the activity of renal PFK-1, which produced the substrate required for ALD activity. However, renal ALD activity was significantly reduced at 20mg/kg DiNP compared to the control, while it increased at 200mg/kg DiNP.
Furthermore, as with ALD, the activities of Lactate dehydrogenase (LDH) and NADase increased at 200mg/kg, as opposed to the decrease observed at 20mg/kg. By reducing NAD to NADH and vice versa, the anaerobic metabolic pathway enzyme LDH catalyzes the reversible conversion of lactate to pyruvate, and its activity serves as an energy homeostasis sensor as well as an index for determining membrane permeability, whereas NADase participates in the calcium signaling pathway as well as the metabolism of nicotinate and nicotinamide. ADP-ribose and nicotinamide are produced by NADase from the substrates NAD+ and H2O. NAD+ also serves as an electron acceptor [30]. From the foregoing, it can be inferred that a rise in the concentration of DiNP may augment the glycolytic pathway, which could ultimately result in the production of energy required for renal function which when produced in excess could also damage renal functions. The tricarboxylic acid cycle is a set of chemical events that occur in the mitochondria of every aerobic organism’s cell to produce energy that is later stored in the form of ATP through the conversion of Acetyl CoA generated from carbs, lipids, and proteins. Acetyl-CoA, a two-carbon molecular compound, and oxaloacetate, a four-carbon compound, combine in a process that is catalysed by CS to create citrate, a six-carbon molecule that releases the CoA group. The emission of a carbon dioxide molecule occurs as a result of the oxidation of isocitrate to the five-carbon molecule known as α-ketoglutarate.
Isocitrate dehydrogenase, another enzyme involved in this process, catalyzes the reduction of NAD+ to NADH. SDH and MDH, on the other hand, use flavin adenine dinucleotide (FAD) as an electron acceptor to reduce ubiquinone to ubiquinol while catalyzing the Krebs cycle’s oxidation of succinate to fumarate [8,31]. DiNP inhibited the activity of several key TCA cycle enzymes, including citrate synthase (CS), isocitrate dehydrogenase (IDH), malate dehydrogenase (MDH), and succinate dehydrogenase (SDH). The activities of these enzymes decreased as DiNP concentration increased in comparison to the control. A mitochondrial enzyme called succinate dehydrogenase supports metabolic activity by way of the TCA cycle and the electron transport chain (ETC). The SDH function in the ETC converts ubiquinone to ubiquinol. The four TCA cycle subunits (SDHA, SDHB, SDHC, and SDHD) that SDH transmits electrons via before continuing this electron transfer as complex II through the ETC. Complex III continues to produce adenosine triphosphate by receiving electrons from FADH2 and reduced ubiquinone [7]. This provides energy to the cell. Cells may carry out cellular respiration, the hypoxic response, and other cellular activities like gene expression thanks to the regulation of this enzyme by its different complexes [31]. The I, II, III, and IV complexes make up this electron transport chain. The tricarboxylic acid (TCA) cycle uses the NAD and FAD-linked enzymes as an electron source and the proton-motive force they produce to produce ATP.
While complex II electrons move to complex III via FAD-linked SDH, complex I electrons move directly to complex III via ubiquinone [8]. In the present study, activities of the ETC complexes were downregulated upon exposure to DiNP relative to the control. The observed down-regulated activities of the complexes may be due to the inhibition of SDH activity, as SDH provides the initial electron transfer for the complexes. Renal Histopathological examination revealed the potential of DiNP to cause distortion of the renal histoarchitecture as evident its ability to induce hyaline degeneration, atrophy of the glomeruli glomerular tissue of indicative of necrotic changes which is also in tandem with the report on DeHP on long exposure disrupting kidney development, arouses glomerulonephritis and damage’s renal function [32-34]. The findings of this study, which provides the first insight into the renal energy metabolism upon DiNP exposure, indicate that DiNP exposure is associated with changes in energy metabolism via perturbations of various energy transduction enzymes in the cytosol and mitochondria. These perturbations epitomize the various level of renal toxicity associated with exposure to DiNP. Taking all aforementioned observations into consideration, this study indicates a possible shift of energy transduction away from TCA and ETC to glycolytic or other possible metabolic pathways as evident in the down-regulation of enzymes of renal TCA and ETC. Since the major energy producing pathway for the kidney is through oxidative phosphorylation, it can be inferred that DiNP inhibited sufficient energy production via the TCA and ETC thereby shifting the energy source to glycolysis which cannot provide sustainable energy for renal function suggesting DiNP could be nephrotoxic.
The authors declare that there is no conflict of interests regarding the publication of this manuscript.


