Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Danielly C Rocha1, Allan Q Garcês-Filho2, Amanda MA Cunha2, Rommel C Monte2, Isadora S Oliveira3, Isabela G Ferreira3, Wuelton M Monteiro4,5, Felipe A Cerni1 and Manuela B Pucca1,2*
Received:February 02, 2023; Published:February 10, 2023
*Corresponding author: Manuela Berto Pucca, Medical School of Roraima, Federal University of Roraima, Av. Capitão Ene Garcez, 2413, Boa Vista, RR, 69310-000, Brazil
DOI: 10.26717/BJSTR.2023.48.007676
Leprosy is an infectious disease caused by a parasite, known as Mycobacterium leprae, able to infect cells from peripheral nerves, leading to a high disabling power due to neural impairment. This pathology is common in more than 120 countries, with more than 100,000 cases occurring every year. Both innate and acquired immune responses are involved, but the disease has been classically described along a Th1/Th2 spectrum, where the Th1 pole corresponds to the most limited presentations and the Th2 to the most disseminated ones. Due to the clinical and histopathological diversity of the disease, the immunopathogenesis of leprosy is not completely understood. Our review describes the role of the immune system in the pathogenesis of leprosy aiming to understand the mechanisms involved in the development of the different immune responses against the disease.
Keywords: Leprosy; Mycobacterium Leprae; Immune Response
Leprosy is an infectious disease with a chronic and slow evolution, manifested mainly by skin lesions with reduced thermal, painful, and tactile sensitivity [1]. It preferentially affects the skin and peripheral nerves located in the face, neck, middle third of the arm, and below the elbow or knees, and can also affect the eyes and internal organs such as mucous membranes, testicles, bones, liver, among others [2,3]. This pathology is considered one of the oldest diseases that affects society [4,5], and holds a terrifying image in the history and memory of humanity based on the oldest reports that considered a contagious, incurable, and disabling disease, leading to the confinement and exclusion of the affected person [6,7].
Leprosy is a neglected tropical disease (NTD), which still occurs in more than 120 countries, with more than 200,000 new cases reported every year, resulting on physical deformity. Stigmatization of persons affected by leprosy continues to hamper early detection, and instances of discrimination against such persons are reported [8]. Leprosy represents a public health problem due to its potential to cause physical, social, and economic disability. In 1991, after the adoption of multidrug therapy as a specific treatment, the World Health Organization (WHO) proposed the elimination of leprosy as a public health problem by the year 2000, with elimination defined as a prevalence of less than 1/10,000 inhabitants [9]. Globally, in 2019, 202,185 new cases of leprosy were detected, within a detection rate of 25.9 per million population. Brazil, India, and Indonesia reported >10,000 new cases, while 13 other countries (Bangladesh, Democratic Republic of the Congo, Ethiopia, Madagascar, Mozambique, Myanmar, Nepal, Nigeria, Philippines, Somalia, South Sudan, Sri Lanka, and the United Republic of Tanzania) reported 1,000 – 10,000 new cases each. Forty-five countries reported 0 cases, and 99 reported <1,000 new cases. The geographical distribution of new cases detected in 2019 is presented in Figure 1 [8], demonstrating the heterogeneous distribution of leprosy in developing countries [10].
Leprosy in Brazil
Brazil is classified as a country with a high leprosy burden, ranking second in the list of countries with the highest number of cases in the world, behind only India. Brazil is responsible for 93% of the cases of the disease that occurred in America, 5.5% of the new cases in 2019 occurred in children under 15 years of age, and 9.9% had G2D among the 23,843 patients evaluated at diagnosis. Among the diagnosed cases, there is a predominance of males, aged between 50 and 59 years, black (followed by whites), and presenting incomplete high school. Covid-19 epidemic influenced the diagnosis and monitoring of leprosy cases in Brazil. Preliminary data for 2020 show that Brazil diagnosed 13,807 new cases of leprosy. Mato Grosso is the state with the highest number of new cases in the general population, 1,853, followed by Maranhão, Pará and Pernambuco, with more than a thousand cases each [11]. In this sense, it is highlighted that the distribution of leprosy in Brazil follows similar distribution of the disease in the world and the detection rate of new cases in 2020 remained indicating that the cases are concentrated in three regions of the country: Midwest, North, and Northeast (Figure 2), according to records from previous years [11]. In 2020, Mato Grosso was the state that had the highest overall detection rate, 71.44 new cases per 100,000 inhabitants; its capital, Cuiabá, recorded a rate of 29.78 cases per 100,000 inhabitants. Tocantins ranked second among Brazilian states, with 53.95 new cases per 100,000 inhabitants, and its capital, Palmas, recorded a rate of 118.51 cases per 100,000 inhabitants, the highest among the country’s capitals. Rio Grande do Sul and Santa Catarina, as well as their capitals, have low endemicity [11]. The incidence is an important parameter as it measures the strength of morbidity, magnitude, and trend of the endemic (Figure 3).
The classification of leprosy cases, based on the number of skin lesions, is given according to the following criteria: Paucibacillary (PB) – cases with ≤5 skin lesions; and Multibacillary (MB) – cases with >5 skin lesions. In Brazil, the proportion of new multibacillary cases was 61.0% in 2011 and 80.1% in 2020, an increase of 31.3%. There was an increase in all regions, with a higher proportion in the Central- West and North regions – 37.9% and 35.8%, respectively [11]. For multibacillary cases, the presence of physical disability is commonly high, a consequence of neural damage during the disease and late diagnosis, which facilitates the maintenance of active cases of the disease and a marker for the recognition of continuous transmission of the infection in the disease community [12].
Leprosy in the Amazon
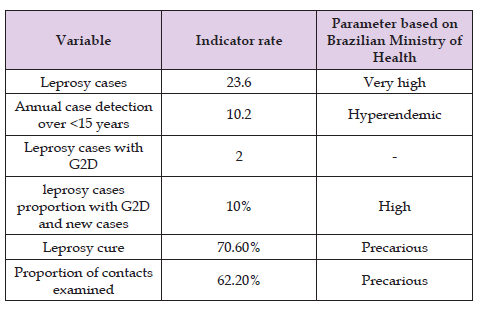
Regarding Brazil, the north region presents the highest number of new leprosy cases. Indeed, the disease has been highly endemic to the Brazilian Amazon for more than 100 years [12]. People living in leprosy-endemic regions are at greater risk of being exposed to the infection. The risk of developing the disease among paucibacillary contacts is 2–3 times higher than that of the general population, while the risk increases to 5–10 times among multibacillary contacts [13]. The most endemic areas in the region are found from the state of Rondônia, through the north and center of Mato Grosso, south of Pará, northwest of Tocantins, to the extreme west of Maranhão [14]. In Belém, capital of Pará, for example, the cases that occurred between 2006 and 2015 were mostly borderline leprosy, followed by tuberculoid and lepromatous forms. In addition, there was a high frequency of multibacillary cases, a finding compatible with a hyperendemic situation. At the time of diagnosis, most had some degree of physical disability, making the late diagnosis evident [15]. Although leprosy affects all ages, children under 15 years of age are an important epidemiological marker for the WHO, which has as one of its goals 90% reduction in the rate per million children of new child cases with leprosy. This disease detection rate in children under 15 years of age in some states of the Amazon is a challenge for the Brazilian government as it remains high. A study conducted in 2017, with 34,547 students from a public school in Manaus, the capital of Amazonas state, shown that the number of cases in this group was 17 times higher than the number recorded by the state, which indicates the high endemicity of the disease in the state [13]. In Roraima, the Northernmost state of Brazil, it was detected 129 cases of leprosy in 2017, with Boa Vista helding 59.4% of these cases, and a detection rate of 23.6 cases per 100,000 inhabitants, which was considered very high by the National Ministry of Health [11]. Thus, in 2017, Roraima presented an alarming situation (Table 1), attesting not only the high rate of involvement, but also deficiencies in the services offered [16].
Table 1. Indicators for monitoring the progress of leprosy in Roraima state per 100,000 inhabitants in 2017.
Leprosy is caused by Mycobacterium leprae, an acidalcohol resistant and slightly gram-positive bacilli belonging to Mycobacteriaceae family, Actinomycetales order, and Schizomycetes class. The cardinal signs of leprosy include sensation loss in skin lesion, enlargement of peripheral nerve and positive skin smears [17]. M. leprae is an intracellular pathogen and shows affinity for neural cells, specifically, the Schwann cells [18]. Due to this reason, it has dermo-neurological involvement, with cutaneous injuries and peripheral nerves, mainly in the eyes, upper and down limbs, characterized by hypochromic spots, infiltration, nodules, and other features [18,19]. M. leprae has slow multiplication rate and perform binary division along 12 to 21 days [20]. Its slow reproduction results in disease chronicity and long period of incubation, varying between 2 to 7 years [21]. M. leprae has high infectivity, but low pathogenicity, for this purpose, it is possible that the individual can activate the own immune system against the pathogen and may not manifest the disease [22]. The M. leprae is transmitted by prolonged contact with untreated patients, by coughing, sneezing, or talking, expelling droplets, which are aspirated by other individuals through the upper airways, contaminating them [23]. Normally, the etiological agent source is a family member who does not know that is contaminated [24]. It is noteworthy that leprosy occurrence does not has a linear reaction (cause and effect), instead it is a multifactorial disease involving physiological (immunity), psychic (depression and stress), cultural (habits and behavior), environmental (little sun light, absent ventilation), and socioeconomic aspects (low education, precarious health conditions, among others) [6,25].
Despite the fact that most of nose’s bacilli is found in macrophages, it is known that they can be also found inside Schwann cells, monocytes, columnar, polymorphs, ducts, and secretory gland [26]. Indeed, the leprosy-induced neuropathy emerges from M. leprae invasion into the Schwann cells. Once in the cell, it provokes a cellular differentiation destroying the myelin sheath, causing an axon loss function, leading to infection dissemination [27]. Another relevant aspect to the leprosy neuropathy development is the immunoinflammatory response to the bacilli presence. Although Schwann cells are not specialized phagocytic cells, they are known to produce TNF-a, which has a pathogenic role in nerve injury [28]. Neural impairment occurs in all clinical manifestation of leprosy [29], affecting peripheral nerves from the cutaneous endings to the nerve trunks, causing clinically mixed neuropathies, derived from the involvement of sensory, motor, and autonomic nerve fibers [30]. Regarding sensory changes, thermal sensitivity is initially corrupted, followed by reduction (and even loss) of painful and touch sensitivity [31]. Motor change leads to paralysis or paresis, weakness, and muscle atrophy. Dry skin and fissures can occur due to secretion decrease that gives flexibility and texture to the skin. These are results of autonomic nerve fiber involvement [29].
Such dysfunctions are frequently accompanied by intense pain, nerves hyper sensibility, motor and sensory deficit, and edema [30]. On the other hand, dysfunctions may be developed without pain, characterizing silent neurites, without nerve hyper sensibility changes, but there is sensibility or motor strength dysfunction [32]. In general, nerve receptors commitment, responsible for touch sensitivity, painful stimulus, and eyesight, makes individuals more vulnerable to accidents, wounds, burn, and even amputation, resulting in psychologic and social damage, which interfere with quality of life [33]. Leprosy neuropathy involves mainly nerves of peripheral face (trigeminal and facial), arms (radial, ulnar and median nerve) and legs (fibular and tibial) (Figure 4). In the face, the lesion of the zygomatic branch causes paralysis of the orbicularis musculature, making it impossible to occlude the eyelids, leading to lagophthalmos. In the upper limbs, the radial nerve injury, the least affected among them, leads to loss of finger and wrist extension, causing a “dropped hand” deformity. In the ulnar nerve, it causes the paralysis of the interosseous and lumbrical muscles of the fourth and fifth fingers of the hand, which leads to an imbalance in flexion and extension of the fingers, thus, the proximal phalanx is hyperextended, and the deep flexors exaggeratedly flex the distal phalanges, resulting in claw hand. The median nerve can be affected in the wrist region, causing paralysis of the thenar muscles, with loss of thumb opposition. In the down limbs, the damage in the tibial trunk leads to claw toe and an important loss of sensitivity in the foot plantar region, resulting in secondary consequences as the plantar ulcers. In the fibular nerve it may occur paralysis of the dorsiflexor and evertor foot musculature and, therefore, unable the foot to be elevated, “foot drop”, which changes the normal gait dynamics [24].
Physical disability may also result in leprosy reactions (reactional
episodes) [34], which consists in exacerbated inflammatory reactions.
They may occur before diagnosis, during or post pharmacological
treatment. There are two types of reactions:
i) Reversal Reaction (or type 1 reaction), characterized by
new skin lesion (spot or plaques) and color changes, infiltration,
pain and edema in preexistent lesions, with or without neural
inflammation and thickening; and
ii) Erythema Nodosum Leprosum Reaction (type 2 reaction),
the most common type, which is characterized by the presence
of painful subcutaneous nodules, may occurring fever, sharp joint
pain, with or without neuritis and thickening [24].
Type 1 reaction is considered the most disabling to the individual due to the presence of acute pain, leading to frequent absences and sick leaves and, consequently, to unemployment [34]. In Brazil, the clinical classification of leprosy is based on the 6th International Congress of Leprosy, held in Madrid (1953) [35]. Cutaneous manifestations are classified as undetermined, tuberculoid, borderline and lepromatous leprosy [24]. Nonetheless, there may occur neural commitment without skin lesion, by which the cases are classified as primarily neural leprosy or pure neural form [36,37], as described:
1) Indetermined leprosy (Figure 5A) is the initial phase that
all the patients present at the beginning of the disease. It can be
imperceptible and evolve to spontaneous cure or to the other
forms (~25% of the cases). Usually it presents a single injury
(stain), clearer, without relief and poorly defined edges. There is
thermal and/or painful sensitivity commitment, maintaining the
tactile sensitivity. There may occur alopecia and lesion dryness
(because there is no sweating in the spot).
2) Tuberculoid leprosy (Figure 5B) is considered the most
benign because the host immune system can destroy the bacillus;
it occurs in people with high resistance to the mycobacteria, which
means that there is a high cell-mediated immunity. It presents
few lesions with little elevation, well delimited and a clear center
(as a circle). The commitment is symmetrical, following the
nerves path, leading to total loss of thermal, tactile, and painful
sensitivity in these areas, which decreases the need of subsidiary
exams. There may occur loss of local sweating and alopecia.
3) Boderline leprosy (Figure 5C) is the most common form of
the disease, corresponding to about 70% of the cases. It shows
similar features to the tuberculoid and lepromatous forms. It
has a variety of skin lesions that are shown as reddish or whitish
plaques (stain), poorly delimited periphery, with raised edges
and a symmetrical tendency. However, the lesions may be like
tuberculoid lesions, well delimited, but with the external edge
poorly defined. There is partial or total loss of sensitivity. The
nerve commitment is commonly asymmetrical and with a high
extension, enabling the emergence of acute neuritis which can be
visible during examination.
4) Lepromatous leprosy (Figure 5D) is the most contagious
form. In this sort of leprosy, immune system action is absent,
propitiating bacillus proliferation and, consequently, high
severity. Can be reported hands and feet anesthesia, and the host
becomes more prone to lesions, trauma, and edema, resulting in
deformities. As to cutaneous commitment, the patient does not
present spots; the skin appears reddened, dry, with infiltrations
and an “orange peel” appearance. Dark, hardened lumps
(hansenomas) are common. Warm areas are generally spared
(armpits, lumbar spine, and scalp); however, it can affect the ears,
nose (congestion), superciliary and ciliary regions (madarosis),
oral mucosa and internal organs (liver, spleen, adrenals, and
testes). The face is usually free of wrinkles (smooth). Careful
assessment of sensitivity changes is important, as the nerve
involvement is symmetrical, and the superficial nerve branches
may present thickening. Indetermined and tuberculoid form
are denominated paucibacillary, which means that the disease
carriers have low bacteria burden, and their immune system is
capable to destroy the pathogen. On the other hand, lepromatous
and borderline leprosy are multibacillary, and the patients show
high bacillus burden and an ineffective immune system, unable
to control the disease, being these patients capable to spread the
disease [38-41].
Another classification that is widely used in the scientific field,
proposed by Ridley and Jopling (1966) [39], considers the host
immunity and resistance. In this classification the indetermined type
is not included, and histopathological exam becomes necessary for its
use. Such criteria classify leprosy in two polar forms:
i) Tuberculoid (TT); and
ii) Lepromatous (LL);
While in indetermined forms:
i) Borderline tuberculoid (BT);
ii) Borderline (BB); and
iii) Borderline lepromatous (BL).
It is considered that BT, BB and BL are immunologically unstable,
and they can present changes for any of the disease poles. On the other
hand, WHO classification, for therapeutic purposes, divides patients
into paucibacillary (PB) and multibacillary (MB) based on the number
of skin lesions. PB cases have up to five skin lesions in total, whereas MB cases have six or more skin lesions. The three systems presented
here are complementary rather than exclusive [40-42].
The immune response is commonly divided into innate immunity and adaptive immunity. Innate immunity consists of physical, biological, and chemical barriers, specialized cells, and soluble molecules, which are present in all individuals, even without previous contact with pathogens or bioagents. The basal effector cells of innate immunity are macrophages (MCs), neutrophils (NT), dendritic cells (DCs), and Natural Killer (NK), responsible for the main mechanisms of the innate response such as phagocytosis, release of mediators pro-inflammatory, activation and synthesis of proteins, cytokines, and chemokines. In contrast, the adaptive immune response depends on the activation of specialized cells (e.g., lymphocytes), and the soluble molecules they produce. It can be categorized into cellular and humoral response, mediated by cells and antibodies, respectively. It is mainly characterized by the specificity of recognition, memory, self-limitation, and tolerance to components of the organism itself [43,44]. Despite efforts to identify cellular and inflammatory markers, the immunopathogenesis of leprosy is not completely understood. This is due to the clinical and histopathological diversity of the disease and the polymorphism of the immune system in the population, evidenced by the presence of mixed cell phenotypes at different steps of infection [45,46]. In this way, the immune response corresponds to an important element in the determination of leprosy clinical manifestations, with apparent polarity between tuberculoid and lepromatous forms [23,47]
Innate Immune Response Targeting M. Leprae
An effective innate immune response is directly related to the low virulence and control of M. leprae [43]. In fact, innate immunity is a non-specific type of defense since marked by different strategies of defense (Figure 6). The innate immunity in leprosy. The complement system is a protein-based mechanism of defense of resulting in the membrane attack complex (MAC) formation (i.e., leading to cell membrane disruption of invading organisms), activating phagocytic cells through opsonization, and inducing inflammation. Antigenpresenting cells (APCs) (e.g., macrophages and dendritic cells), are capable of promoting phagocytosis of M. leprae, resulting in the presentation of the bacillus-derived antigens to T lymphocytes, being responsible to induce the adaptative immunity. Natural killer cells (NKs) are also important to control the disease at early stages of the immune response. Regarding temperature, pH, and physical barriers, they are the body’s natural defenses against any external microorganisms.
The epithelial barrier (i.e., epidermis) plays an important role in the innate immune response against leprosis. The keratinocytes, the most common cells of the epidermis, can release different cytokines when stimulated, including TNF-a, IL-6, and IL-12, as well as show high expression of ICAM-1 (in tuberculoid, but not in lepromatous leprosy). In addition, the epidermis also acts as a physical barrier, preventing the route of microorganisms [48,49]. The complement system (CS) compromises more than 30 serum and cell‐associated proteins and plays an important role in host immunity against leprosy. Indeed, MAC activation or the soluble terminal complement complex (TCC) were demonstrated in association with damaged nerve in leprosy patients [50]. Nevertheless, additional studies are still necessary to elucidate the participation of the CS in the pathophysiology of the disease, although it is clear the systemic activation of complement which probably involves multiple pathways [50]. The M. leprae is known to be recognized by Toll-like Receptors (TLRs). TLRs are cellular receptors present mainly in antigen-presenting cells (APCs), which are responsible for recognizing different types of antigens. The TLR2- TLR1, TLR2, and TLR4 were identified as the main Toll-like receptors able to recognize M. leprae antigens [51,52], although recently (2021) TLR9 demonstrated to also sensing M. leprae DNA [53]. In fact, phagocytes from the nasal mucosa (MCs and DCs) are known to be the first cells from immune system that recognize M. leprae by TLRs [48,54], once the pathogen is dispersed mainly through the upper airways and notably through the nose.
Furthermore, neutrophils are also indicated as important in the innate defense against leprosy. Although little is still known regarding the NTs role in the clinical manifestations, it is known that these cells present divergent phenotypes that are dynamic subpopulations with distinct phenotypical and functional abilities, which could indicate them as a clinical biomarker in the future [55]. Regarding macrophages, they can be classified into M1 and M2. The M1 type is related to inflammatory and antimicrobial activity, and it is activated by pro-inflammatory cytokines, such as INF-y. In contrast, M2 is activated by IL-4 and IL-13, presenting anti-inflammatory activity, most related to tissue repair [56]. Previous studies have shown that in a patient with leprosy, the two types of macrophages coexist in the inflammatory environment, together with different DC subsets. Still, other studies indicate that according to the classification of leprosis can be observed different macrophages cells populations, with predomination of M1 cells (paucibacillary lesions and in the onset of reversal reaction) or M2 cells (lepromatous skins tissue) [48,57]. Thus, macrophages play critical roles in modulating M. leprae infection. Finally, NKs are also considered key cells on the innate immunity against leprosy since protection against intracellular such as M. leprae is critically dependent on the function of these cells at early stages of the immune response and on Th1 cells at later stages [58].
Acquired Immune Response Targeting M. Leprae
Following M. leprae phagocytosis, APCs present the antigen to a naive T cell, resulting in its activation. During this process, APC releases IL-12, which normally (90% of the population present in the natural resistance and TT) leads to an expansion of Th1 cells, responsible to produce M. leprae “kill factors”. Therefore, in TT, there is an intense cellular response mediated by Th1 lymphocytes that produce pro-inflammatory cytokines (e.g., IL-2, IFN-γ, and TNF-α) that control bacterial growth at the site of infection. These cytokines work together to recruit and activate NTs and monocytes that fight bacterial growth and multiplication at the infection site [22,59] (Figure 7). In the case of Th1-trigered leprosy response, the presence of specific antibodies is weak or absent [60]. In contrast, the remaining 10% of the population have a different immunity manifestation. When the natural immunity against M. leprae failures, the body may polarize to a Th2 response instead of Th1 resulting in an anti-inflammatory profile, characterized by decreased cellular response, no granuloma formation, and increased bacillary load, characteristic of the LL leprosy (also known as Virchowian form) [61]. The anti-inflammatory profile is caused by the expression of different cytokines, such as IL-4, IL-5, IL-10 and IL-13 (41). As a result, IL-4 decreases TLR2 expression, and IL-10 suppresses IL-12 production, which is associated with the predominance of CD8+ lymphocytes into lepromatous lesions. In addition, cytokines produced by Th2 profile are known to maintain an anti-inflammatory environment, justifying the failure to reduce the M. leprae growth [22,47]. Due to the CD8+ predominance as well as plasma cells, in LL leprosy the immunologic response mediated by Th2 cells has high titles of antibodies instead of a specific cellular immunity [62].
In summary, the Th1 immune response is recognized to be effective in destroying the bacillus, conferring resistance to the disease, which is tissue localized. On the other hand, the Th2 immune response is not effective to eliminate the bacillus, as it induces high titles of specific antibodies, which are ineffective against intracellular microorganisms, resulting in susceptibility to present the disseminated leprosy type [62]. Although the Th1/Th2 leprosy immune model is well accept by scientists and physicians, reproducing the clinical evidence similar to other diseases such as allergies, with the development of new immunological pathways, today we can find different interpretations in the literature. For instance, some patients demonstrated polarizing to a Th17 response, which promoted the control bacillary load [63]. The disease control may be justified since Th17 cells release IL-17A, IL-17F, IL-21 and IL-22, cytokines that induce inflammatory responses, neutrophil recruitment, macrophage activation, and increase in Th1 effector cells [22]. Other studies have also demonstrated the Th17 involvement in leprosy. Saini et al. (2013) shown that Th17 cells are more associated with tuberculoid form in both skin lesions and M. leprae cultures, suggesting its differential role in tuberculoid and lepromatous leprosy patients [63]. Quaresma et al. (2015) show reduced expression of IL- 17A in leprosy skin as compared to healthy subjects and attributed it to genetic differences [64]. In addition, the role of regulatory T cells (Treg) has also been explored in leprosy. The IL-10 produced by Treg cells has been reported as a suppressive modulator in nature in leprosy [65]. In this scenario, over three decades, Th1 and Th2 paradigm were thought to underline tuberculoid and lepromatous disease respectively. Conversely, today, leprosy is recognized as an infection with a complex immune response, specially concerning the acquired immunity [66,67].
We thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation, scholarships to ISO No. 2020/13176-3 and No. 2022/08964-8), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Coordination for the Improvement of Higher Education Personnel, Finance Code 001, scholarships to IGF), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, The National Council for Scientific and Technological Development, scholarship to MBP no. 307184/2020-0 and WMM n. 309207/2020-7), and PROPESQUISA/PRPPG-UFRR (n. 06/2021).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.


