Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Gianne Camille Zozobrado de Castro1* and Raymond L Rosales2
Received: January 25, 2022; Published: February 07, 2023
*Corresponding author: Gianne Camille Zozobrado de Castro, Resident Physician , Department of Neuroscience and Behavioral Medicine, University of Santo Tomas Hospital, Philippines
DOI: 10.26717/BJSTR.2023.48.007653
Background: Myofibrillar myopathy (MFM) is a neuromuscular disorder, usually with adult onset and an autosomal dominant inheritance pattern, that results in slowly progressive weakening of limb muscles [1]. MFM may lead to multisystem involvement (eye, peripheral nerve, and heart), and progressive cardiorespiratory complications are potentially lethal [1]. The prevalence of this condition is not exactly known [2]. To our knowledge, no such report has been made in Southeast Asia. Thus, we aimed to report such a first case in our locale.
The Patient: The phenotype is a 69-year-old Filipino Chinese with short stature, who presented with a slowly progressive proximal-dominant muscle weakness involving lower limbs initially within 2 years. The clinical examination revealed a short stature percentile having proximal-dominant muscle weakness but involving distal lower limbs as well. He had hyporeflexia, slightly weak lateral rectus eye muscles and ataxia. Cardiac arrhythmia was documented earlier necessitating a pacemaker placement and obstructive sleep apnea requiring assisted sleep ventilation. The family history was not yielding, and among siblings, he was the only one with short stature.
Conclusion: From our locale and Southeast Asia region, we document for the first- time case of MYH2 Myopathy, consistent with the phenotype reported from elsewhere having myopathy, neuropathy and eye movement disturbance. Whether in fact our new interesting findings of cardiac arrhythmia, obstructive sleep apnea, and short stature in this present case report, be part of the MYH2 disease spectrum, will require further investigation.
MYH2 myopathy belongs to the category of muscular dystrophies. Primarily affecting skeletal muscles. In some cases, the heart (cardiac) muscle may also be involved [3]. To our awareness, this is the first case report of MYH2 Myopathy found in our locale.
The patient is a 69-year-old, Filipino-Chinese male, who presented with a progressive, proximal-dominant muscle weakness of the lower extremities within a span of 2 years. His physical examination revealed that he had short stature (below average percentile), bilateral lateral recti muscle weakness, gait ataxia and hyporeflexia. Relevant past medical history includes an implantable cardioverter defibrillator (ICD) for arrhythmia and obstructive sleep apnea requiring positive airway pressure ventilation when sleeping. His family history was unremarkable for a neuromuscular disease. There was consanguinity of parents.
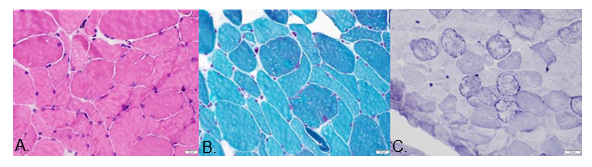
From elsewhere performed, electrodiagnosis showed symmetric axonal neuropathy with reduced CMAPs and SNAPs. Sural nerve biopsy documented axonal degeneration without demyelination and vasculitis. Search for metabolic disease, including a malignancy scan were not yielding, and so were the autoimmune serologic tests. (Table 1). Total CK we tested was not elevated, but due to dominant proximal weakness, a quadriceps biopsy was performed: myofibrillar myopathy pathology with some neurogenic changes. Moving to genetic testing, we verified the presence of heterozygous variant of MYH2 missense mutation by clinical exome sequencing. The patient was put on rehabilitation care, given symptomatic treatment for pain and ensuing depression apart from onboard cardiopulmonary drugs. The medications given were as follows: Trimetazidine HCl, Atorvastatin, Losartan, Ticagrelor, Tauroursodeoxycholic Acid, Coenzyme Q10, Pyrroloquinoline quinone. Patient’s somatosensory evoked potential revealed symmetrical distal neuropathy affecting the lower extremities. Snap frozen cryostat sections of muscle revealed mild to moderate variation in fiber size with few angular atrophic fibers and rare necrotic and regenerating fibers. Moth-eaten fibers were notable on NADH-TR histochemical stains. No raggedred nor rimmed vacuoles were found in Modified Gomori Trichome stain. Muscle fiber glycogen (PAS stain) and lipid contents (Oil-Red-O stain) were unremarkable. (Table 1) Myofibrillar myopathy was the histopathologic diagnosis.
Total Sural Nerve Biopsy: Plastic paraffin embedding and staining cross sectional with Gomori-trichrome showed axonopathy, as follows: In all nerve fascicles, there were reduced myelinated nerve fiber population, and myelin ovoids. Pathological diagnosis: Axonal degeneration.
Quadriceps Open Muscle Biopsy: Snap freezing with dry-iceacetone technique, followed by cryostat cross sections.
HE Stain: Muscle fiber variability; occasional presence of angular fibers; no necrotic fibers nor inflammatory mononuclear cell infiltrates.
Modified GOMORI-Trichrome stain: Slight muscle fibrosis; “no ragged-red” fibers. No rimmed vacuoles.
NADH-TR Stain: showed “moth-eaten” fibers and some lobulated fiber appearance; lightly stained type 2 muscle fibers have reduced diameter; some angular fibers were noted and Type-2 muscle fiber sizes appeared reduced. Pathological Diagnosis: Myofibrillar myopathy and neurogenic changes; Type-2 fiber atrophy (Table 2).
We have a 69-year-old male who had a progressive proximaldominant lower extremity weakness with ophthalmoplegia, gait ataxia and hyporeflexia. He had a pacemaker insertion due to cardiac arrhythmia at an early age, was diagnosed with obstructive sleep apnea. Histopathologically, our patient’s sural nerve biopsy showed axonopathy while muscle biopsy is consistent with myofibrillar myopathy. (Figure 1) Clinical exome sequencing (CentoDX) + CNV analysis revealed a heterozygous variant of MYH2 missense. Three articles of MYH2 myopathy were reviewed for comparison based on the clinical findings as well as the CK levels, genetic analysis, nerve and muscle biopsy. The predominant symptoms of the 11 patients were ophthalmoplegia, proximal and distal limb weakness, facial weakness, cardiac and pulmonary symptoms. Myosin heavy chain (MyHC) is a major structural component of the striated muscle contractile apparatus [4]. Myosin myopathies have emerged as an important group of diseases with variable clinical and morphological expression dependent on the mutated isoform and also the type and location of the mutation [4]. The major feature for MYH2 associated myopathy is ophthalmoplegia and is reported both with recessive and dominant inheritance. Other features are proximal and sometimes distal weakness and joint contractures but with no clear correlation depending on mode of inheritance [4-6]. Clinical and morphological phenotypes are continuously updated by new reports [3]. Aside from the common abovementioned findings, we also uncovered the unique feature of obstructive sleep apnea and short stature that may be a characteristic yet to be discovered part of the MYH2 disease spectrum (Figure 2).
Figure 1:
A. Figure A: HE stain.
B. Figure B: Modified Gomori-Trichrome stain.
C. Figure C: NADH- TR stain.
We document for the first time, a case of MYH2 Myopathy from our locale and Southeast Asia, consistent with myopathy, neuropathy and oculomotor disturbances. New findings of obstructive sleep apnea and short stature were present in this case and may be part of the MYH2 disease spectrum. However, due to limitations of the said data, further investigation is needed.
This case report has been approved by the Research Ethics Committee of the University of Santo Tomas Hospital, as required by the institution for presentation.
A written informed consent for writing and publication of this case report was obtained from the patient’s wife. This is due to the patient’s incapability of writing as a result of his current medical condition. History taking and videotaping was done during physical examination. Data from his follow up including an array of ancillaries were also gathered. All information including videos regarding the patient was kept in strict confidence and patient identifiers were not included in the manuscript. Patient’s anonymity and confidentiality is protected by non-disclosure of any personal information that will identify the individual when the study is published or presented. A breach of confidentiality may occur if the information is used in any other way. As a preferred language by the patient and his wife, the Informed Consent Form written in English was used.
There will be no benefits directly to the patient but will help future physicians in management of future cases.
No potential conflict of interest relevant to this article was reported by the investigators that may interfere with the presentation, review or publication of this case.
Videos of the patient’s disability during actual physical examination will not be included in any presentation. Mr. Murillo is a private patient of the co-author of this paper, Dr. R. Rosales. The principal investigator of this paper, was able to obtain a proper informed consent without influence from the private physician through a private meeting between the patient, the responsible caregiver which is the wife, and myself. The informed consent was thoroughly explained to the patient and his wife, and it was emphasized that they have the freedom and the right not to sign the consent if they chose not to participate in this case report. He was reassured that no consequence would result from him not signing the consent and that he will not be treated otherwise by his attending physician even if he refuses to sign the consent form. The patient acknowledged this and that he fully understood the informed consent and he freely volunteered to sign the waiver after reading it with his wife.


