Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Genanew Kassie Getahun1,2*, Yonas Benti2, Fekede Woldekidan3, Tewodros Shitemaw1,2 and Zelalem Negash4
Received: December 13, 2022; Published: January 10, 2023
*Corresponding author: Genanew Kassie Getahun, Kotebe Metropolitan University, Menelik II Medical and Health Science College, Addis Ababa, Ethiopia
DOI: 10.26717/BJSTR.2023.48.007587
Background: Hypertension in pregnancy is considered a systolic blood pressure of 140 or above, a diastolic blood pressure of 90 mmHg, or both. In Ethiopia, 10% of maternal deaths are brought on by pregnancy-induced hypertension. The objective of this study was to determine the prevalence and associated factors of pregnancy-induced hypertension among women receiving antenatal care in Addis Ababa, Ethiopia.
Methods: A facility-based cross-sectional study, including 313 expectant mothers, was carried out. Data were gathered using a pre-tested, standardized questionnaire. The datasets were double-checked for correctness and exported to SPSS for analysis. To determine the independent causes of pregnancy-induced hypertension, a logistic regression analysis was utilized, and a P-value of less than 0.05 was used to determine statistical significance.
Result: Across the board, the prevalence of pregnancy-induced hypertension was 11.5% (95% CI: 7.7–15). Moreover, the independent predictors of pregnancy-induced hypertension include maternal age (AOR = 3.15, 1.13, 8.79), diabetic mellitus (AOR = 7.35, 1.79, 30.02), chronic hypertension (AOR = 3.26, 1.17, 9.06), family history of pregnancy-induced hypertension (AOR = 4.18, 1.37, 12.77), and history of kidney disease (AOR = 3.62, 1.21, 10.88).
Conclusion: Compared to the Ethiopian national emergency obstetric and newborn care report, the prevalence was high. Also, it was discovered to be associated with a history of diabetes mellitus, renal disease, and chronic hypertension. To ensure early detection and treatment of pregnancy-induced hypertension, special attention should be given to pregnant women with this disease.
Keywords: Prevalence; Pregnancy-Induced Hypertension; Antenatal Care; Associated Factors; Addis Ababa
Abbreviations: AOR: Adjusted Odds Ratio; ANC: Antenatal Care; COR: Crude Odds Ratio; CI: Confidence Interval; HDP: Hypertensive Disorders of Pregnancy; PIH: Pregnancy- Induced Hypertension; SD: Standard Deviation
A systolic blood pressure of 140 or above, a diastolic blood pressure of 90 mmHg, or both, is hypertension in pregnancy. Diagnosing hypertension disorder of pregnancy (HDP) requires taking two or more consecutive measurements with elevated systolic and diastolic blood pressure [1]. Pregnancy-induced hypertension (PIH) includes pre-eclampsia, eclampsia, gestational hypertension, and chronic hypertension [2]. Pregnancy-induced hypertension (PIH), also referred to as toxemia, is hypertension that appears after 20 weeks of pregnancy in a pregnant woman whose blood pressure had previously been normal. Pregnancyinduced hypertension is one of the main causes of death and morbidity and affects 5–10% of all pregnancies worldwide [3,4]. Pregnancy-induced hypertension is a significant public health problem [2]. It affects 2.73% of women worldwide, particularly eclampsia, chronic hypertension, and preeclampsia, which affect 0.28%, 0.29%, and 2.16% of pregnant moms, respectively [5]. Over 830 women’s deaths every day in 2015 were reported because of problems associated with pregnancy and childbirth [6]. The bulk of these deaths almost all occurred in low-resource nations, which could have been avoided [6,7]. Women with PIH are five times more likely to experience perinatal death compared to women without the condition [2].
Compared to women in affluent countries, mothers in poor nations had a thirty threefold higher risk of dying during pregnancy [8]. 1.8 to 16.7% of pregnancies in developing nations are affected by pregnancy-induced hypertension, and more severe cases are linked to poor fetal and maternal outcomes in both industrialized and developing nations [9-12]. Every year, around 350,000 women die worldwide, with roughly 12% of these deaths due to pregnancyrelated causes, and more than half of these deaths happen in sub-Saharan Africa [13]. The prevalence of pregnancy-induced hypertension ranges from 1.8% in the Middle East to 4.5% in the American regions [14]. However, it is estimated to be more common in Africa, affecting one out of every ten women [15]. There have been reports of risk factors for pregnancy-induced hypertension, including maternal age, marital status, education, parity, gravidity, gestational age, history of pregnancy-induced hypertension, family history of such a condition, history of diabetes mellitus, salt consumption, history of chronic hypertension, history of renal disease, history of smoking, history of recurrent miscarriages, and history of excessive alcohol consumption [16-21].
In Ethiopia, 10% of maternal mortality is caused by pregnancyinduced hypertension [22]. Although the Ethiopian government has made significant progress in providing maternal health care services, maternal morbidity and mortality are still high, and pregnancy-induced hypertension is the primary cause [23-25]. This is true even though most deaths caused by PIH can be prevented by providing timely and appropriate treatment of complications. Therefore, the aim of this study was to assess the prevalence of pregnancy-induced hypertension and associated factors among women receiving antenatal care in public health centers in Addis Ababa, Ethiopia.
Study Area and Period
The study was conducted in Addis Ababa, Ethiopia. Addis Ababa is the capital city of Ethiopia. Administratively, it is divided into eleven sub-cities, and it has a total of 56 hospitals (of which 14 are public) and 99 health centers. The city administration had an estimated total population of 5,005,524, where 7.16% of them were children under-five years of age [26].
Study Design and Population
The study population consisted of randomly chosen pregnant women who came for prenatal care follow-up and was studied using a facility-based cross-sectional study design.
Sample Size Calculation
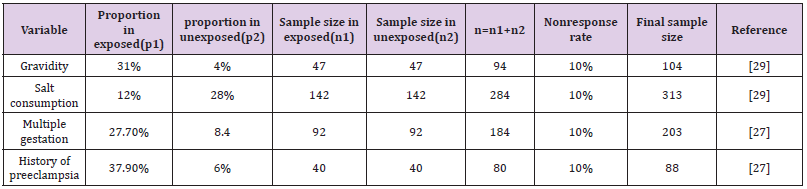
The sample size was first determined using a single population proportion formula with a 95% confidence level, a 4% margin of error, and a prevalence of pregnancy-induced hypertension of 10.3% [2], which gave 216. However, Using EP INFO version 7 and the assumptions of a 95% confidence level, 90% test power, and a 1:1 ratio, we determined the maximum sample size (Table 1). Finally, the maximum sample size calculated for this study was found to be 313, which was larger than the sample sizes determined for any other variable. It was established for the related factor salt consumption components. As a result, the ultimate sample size for this study was 313 individuals.
Table 1: Determination of sample size for significant factors identified in previous literature on the study of pregnancy-induced hypertension and associated factors among women receiving antenatal care.
Sampling Procedure
Five of the eleven sub-cities were selected at random using a straightforward process. Then, from the five chosen sub-cities, ten health centers were chosen using a simple random sampling technique, with the total number of health centers in each subcity serving as the sampling frame (kotebe, yeka, shiromeda, hidasse, lomimeda, keranio, arada, churchill, woreda amest, and woreda hulet health centers). Finally, samples were distributed proportionally according to the number of antenatal care followers.
Variable under Investigation
Dependent Variable: Pregnancy-induced hypertension (yes/no).
Independent Variables: Socio-demographic variables: age, marital status, religion, educational status, and occupation. Obstetric and gynecological factors include the following: the number of children, the number of pregnancies, the gestational age, a history of pregnancy-induced hypertension, an abortion history, and a history of multiple pregnancies. Factors connected to health and family history include diabetes, kidney disease, high blood pressure, and histories of those conditions in both the individual and the family. Personal risk factors include drinking alcohol, having a history of chewing tobacco, eating salty foods, using caffeine, and having any relatives who have ever smoked cigarettes.
Operational Definition
Pregnancy-induced hypertension (PIH) is defined as a pregnant woman who is receiving antenatal care and whose blood pressure is 140/90 mmHg at 20 weeks of gestation, as recorded by trained data collectors twice, six hours apart, with or without proteinuria, which includes gestational hypertension, pre-eclampsia, and eclampsia [3]. Pre-eclampsia was defined as occurring after 20 weeks of pregnancy in a previously non-hypertensive woman with proteinuria of 1+ or 2+ on a dipstick if her systolic blood pressure reached 140 mmHg or her diastolic blood pressure reached 90 mmHg [27].
Alcohol consumption: light-moderate = 2 drinks/day (30 ml ethanol).
Excessive = three drinks per day (45 ml of ethanol) [28].
Smoker: someone who smokes one or more cigarettes daily
Nonsmokers are those who don’t smoke cigarettes every day, including ex-smokers [28].
Data Gathering Techniques
The study participants’ sociodemographic, obstetrical, medical disease, and behavioral features that can increase their risk of high blood pressure and related disorders were evaluated using questionnaires. All responders had their height, weight, and blood pressure measured using standardized, calibrated equipment.
A structured questionnaire together with objective measurements of the subjects’ height, weight, and blood pressure were used to gather the data (BP). Three clinical nurses and one supervisor (a health officer) were hired for this project. Data collectors received a full day of instruction on measuring techniques and interviewing strategies.
Quality Control
To ensure that all participants could understand it, the questionnaire was originally written in English before being translated into Amharic and then back into English for the purposes of analysis. Five days before the start of the real data collection, a pre-test was administered to 5% of the study participants. The outcome was left out of the primary investigation. The question was altered considering the results of the pretest. The lead investigator and supervisors checked the completed questionnaire each day, and the data collector was under daily supervision.
Data Analysis and Management
After data collection, it was entered, cleaned up, and edited in EPI INFO version 7.5.1 before being exported to SPSS version 26 for additional analysis. For the description of sociodemographic and other descriptive parameters, frequency and proportion were computed. The continuous variables were described by a mean and a standard deviation. The association between the dependent and independent variables was evaluated using bivariate and multivariable logistic regression. To determine which independent variables were linked to pregnancy-induced hypertension, a binary logistic regression analysis was performed. Variables with a p-value of less than 2.5 were chosen as potential multivariable logistic regression model candidates. Finally, using an odds ratio with a 95% confidence interval, factors having a p-value of less than 0.05 during the multivariable analysis were deemed significantly linked with pregnancy-induced hypertension.
Ethical consideration
The ethical approval and clearance were obtained from the Research and Ethics Review Board of Yanet Health College, and the Addis Ababa Public Health Research and Emergency Management Directorate provided their permission and clearance for the ethical standards. The medical director of each health center was contacted, and consent was sought. Each study participant provided written informed consent prior to the interview. Following completion of all essential explanations regarding the study’s goal and the respondents’ right to confidentiality, the review was restarted. The study’s participation was completely voluntary, and participants were free to leave at any time.
Socio-Demographic Characteristics of the Study Participants
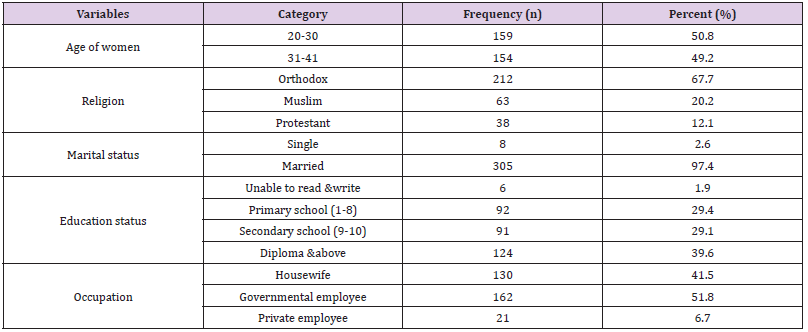
A total of 313 expectant moms took part in the study, yielding a 100% response rate. The respondents’ mean age, which ranged from 21 to 41 years old, was 29.39 + 4.66. Most participants (159, or 50.8%) were between the ages of 20 and 30. Mothers were married in the majority (97.4%) of cases. Most respondents (118, or 39.6%) had a diploma or higher, and 162 (51.6%) of the study’s participants were government workers, followed by 130 (41.6%) housewives (Table 2).
Table 2: Shows the sociodemographic breakdown of the study participants in Addis Ababa, Ethiopia (n = 313).
Obstetrical and Gynecological Conditions
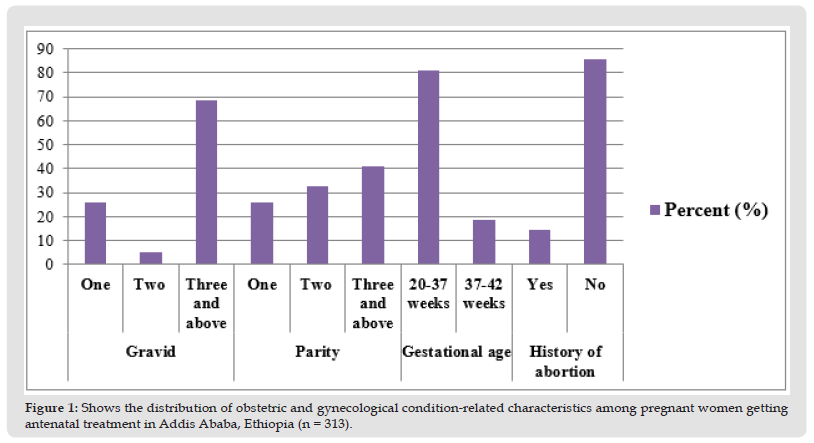
45 (14.5%) of the total study participants had previously undergone an abortion, but none of them had previously had multiple pregnancies. In terms of parity, most women (41.2%) had three or more children, and 250 respondents (81.2%) had gestational ages between 20 and 37 weeks. In terms of the mothers’ gravidity, 214 (68.4%) of them were multigravida. None of the study subjects had previously disclosed having had multiple pregnancies or having experienced pregnancy-induced hypertension (Figure 1).
Figure 1 Shows the distribution of obstetric and gynecological condition-related characteristics among pregnant women getting antenatal treatment in Addis Ababa, Ethiopia (n = 313).
Family History and Past Health
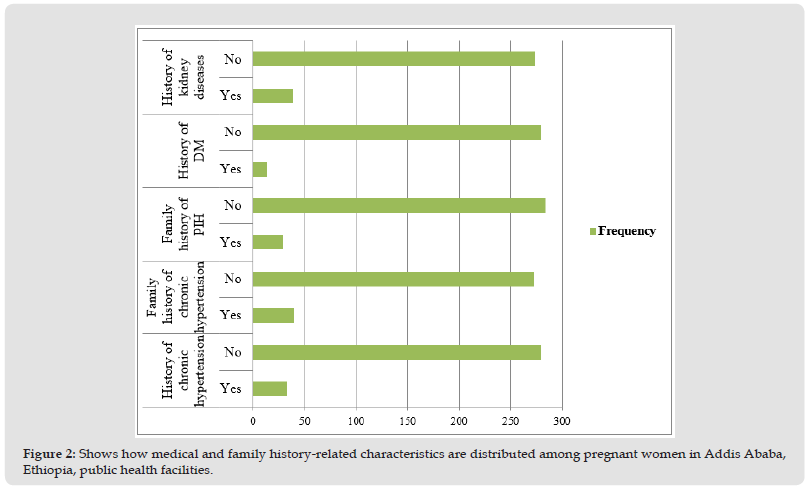
Only 33 moms (10.5%) and 40 women (12.8%) of them had a history of persistent hypertension in their families. Additionally, 39 (12.5%) of the respondents had a history of renal disease, and 29 (9.3%) of them had a family history of pregnancy-induced hypertension. In addition, only 14 mothers (4.5%) had a history of diabetes mellitus (Figure 2).
Figure 2 Shows how medical and family history-related characteristics are distributed among pregnant women in Addis Ababa, Ethiopia, public health facilities.
Behavioral Elements
None of the respondents had ever smoked cigarettes before. However, only 33 (10.5%) of mothers reported drinking alcohol in the previous two years, whereas 36 (11.5%) of participants had a history of passive smoking from family members. 30 (93.8%) of these had consumed alcohol both before and during pregnancy. Most of the pregnant women (309, or 98.7%) who consumed coffee and tea did so while they were pregnant. 264 study participants, or 84.3%, reported consuming salt in their diet. In addition, only 5 (1.6%) of women did not consume a varied diet while they were pregnant (Table 3).
Table 3: Participant distribution for the study by individual risk variables at public health facilities in Addis Ababa, Ethiopia.
Prevalence of Pregnancy-Induced Hypertension
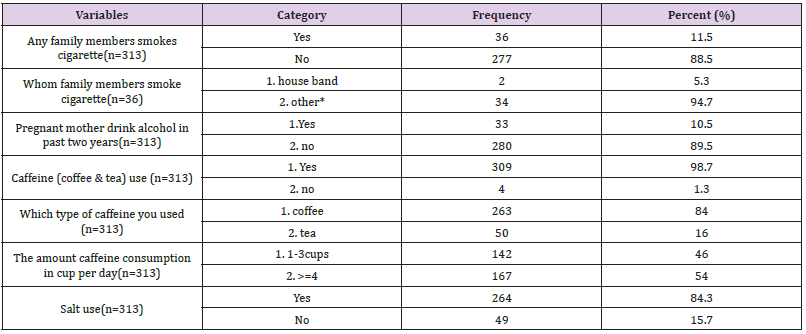
The prevalence of PIH was found in 36 (11.5%) [95% CI: 7.7– 15] of the present study participants getting prenatal care services. Systolic blood pressure values ranged from 110 mmHg to 160 mmHg to 125.91 mmHg on average with an SD of 7.672 mmHg. Additionally, the mean diastolic blood pressure was 79.23 mmHg with an SD of 4.809, the minimum diastolic blood pressure was 70 mmHg, and the maximum was 110 mmHg (Figure 3).
Figure 3 Shows the prevalence of pregnancy-induced hypertension among women receiving antenatal care in public health centers in Addis Ababa, Ethiopia.
Bivariate Association of Different Factors with Pregnancy- Induced Hypertension
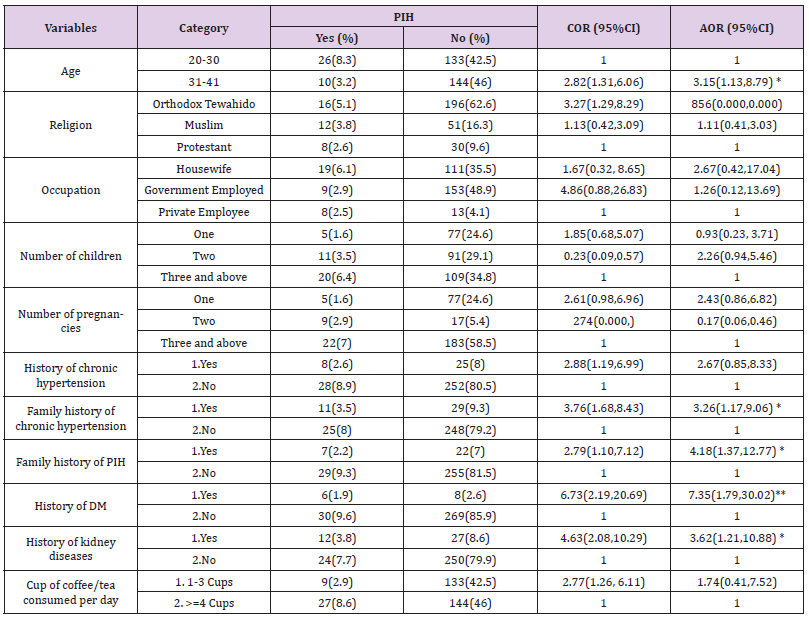
With a P-value of less than 0.25, pregnancy-induced hypertension was statistically associated with age, religion, occupation, number of kids, pregnancy, number of cups of coffee or tea consumed daily, family history of PIH, family history of chronic hypertension, personal history of chronic hypertension, history of kidney disease, history of diabetes, and history of kidney disease (Table 4). In addition, the multivariable logistic regression analysis revealed that maternal age greater than 30 years AOR = 3.15, 95% CI (1.13, 8.79), history of diabetes (AOR = 7.35, 95% CI (1.79, 30.02), family history of chronic hypertension (AOR = 3.26, 95% CI (1.17, 9.06), and family history of pregnancy-induced hypertension (AOR = 3.26, 95% CI (1.17, 9.06)) were the independent predictors (Table 5).
Table 4: Table 5: Results of a multivariable logistic regression analysis for factors related to pregnancy-induced hypertension in women getting antenatal care in public health facilities in Addis Ababa, Ethiopia.
The current study’s findings showed that 11.5% of pregnant women receiving prenatal care in Addis Ababa public health clinics had pregnancy-induced hypertension. The outcome was consistent with earlier studies from Dessie, Ethiopia (8.8%) [25], Jimma, Ethiopia (10.3%) [3], Metu, Ethiopia (12.4%) [29], and Tamil Nadu, India (10.4%) [30], among others. It was marginally higher than the global prevalence of pregnancy-induced hypertension (5–8%) [11], a report from Bangladesh reported 7.5% [31], and a survey from all over Ethiopia reported 6.07% [32]. The difference in research duration, the quality of antenatal care services in diagnosis and management across the various study areas, or the location of study participants could all be contributing factors to this discrepancy. As a result, all our participants were from urban areas and may have better healthcare-seeking habits.
However, when compared to research data from the Northwest of Ethiopia (16.8%) [33], Harare, Zimbabwe (19.4%) [34], and Legos, Nigeria (17%) [35] the study finding was determined to be lower. The variation in the study site, sociodemographic, and methodological approaches may be the cause of this mismatch. For instance, a study in Northwest Ethiopia had 422 respondents from rural and urban areas, while a longitudinal study in Nigeria followed 216 participants during pregnancy, delivery, and six weeks after giving birth, potentially increasing the number of study participants with PIH. Furthermore, it was discovered that pregnancy-related hypertension was significantly correlated with mother age. When compared to respondents who were younger than or equal to 30, those who were older than or equal to 31 had a threefold increased risk of developing pregnancy-induced hypertension. This result is consistent with research done in several regions of Ethiopia [25,36,37]. This could be explained by the fact that older women are more likely than younger ones to have cardiovascular diseases and vascular damage. In addition, as a woman gets older, her body will be less able to digest dietary salt, she won’t exercise, and her body will be less able to process it altogether. This leads to diminished blood vessel flexibility, which ultimately results in hypertension.
This study found that, compared to controls, people with a family history of chronic hypertension and those with a family history of pregnancy-induced hypertension had odds of developing pregnancy-induced hypertension that were roughly three and four times higher, respectively. This agrees with research from Ethiopia [3,32], Nigeria [35], and a book on obstetrics and gynecology [38]. This could have happened because of hereditary components that support the physiologic tendency to pregnancy-induced hypertension. According to this study, women who have a history of diabetes mellitus have roughly seven times the risk of developing pregnancy-induced hypertension compared to those who don’t. It was consistent with research done in Nigeria [35] and Ethiopia [25,29,39]. This could be a result of the shared metabolic and cardiovascular risk factors between diabetes and hypertension diseases. This study also identified that pregnant women with a history of renal disease had a more than threefold increased risk of developing pregnancy-induced hypertension compared to those without such a history. Studies carried out in several parts of Ethiopia [3,39] as well as the United Kingdom [40], provided support for it. This suggests that renal physiologic function and cardiovascular diseases are directly correlated, and that preexisting renal disease has a substantial correlation with pregnancy-induced hypertension.
The primary drawback of the study was its cross-sectional design, which made it impossible to demonstrate a causal link between many independent variables and the outcome variable. The study’s other potential limitations might result from the participants’ ability and willingness to provide correct information about themselves and their family’s history of pregnancy-induced hypertension. Biases might be introduced while collecting data from pregnant women. Steps have been taken to lessen these restrictions, though, by employing focused queries.
The results of this study indicated that, when compared to other studies carried out in Ethiopia [3,9,27,32,37], the prevalence of pregnancy-induced hypertension among pregnant women getting antennal care services was high. Predictors of pregnancyinduced hypertension included maternal age, a history of diabetes mellitus, a family history of chronic hypertension, a family history of pregnancy-induced hypertension, and a history of renal disease.
Consent for Publication
Not applicable.
Data Availability
The corresponding author can provide the datasets that were utilized to support the study’s conclusions upon request.
Potential Conflict of Interest
The author affirms that they have no competing interests.
Funding
The study has no funding source.
Acknowledgement
We would like to extend our sincere appreciation to the data collectors and study participants.


