Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Stefania Murzilli1*, Silvia D’Alessio2, Stefano Lamberti1 and Arianna Vanelli1
Received: December 23, 2022; Published: January 10, 2023
*Corresponding author: Stefania Murzilli, Research and Development, Nutrilinea SRL, part of Biofarma Group, Italy
DOI: 10.26717/BJSTR.2023.48.007584
Keywords: Intestinal Inflammation; Nutraceutical Compounds; Irritable Bowel Syndrome
Acute and chronic inflammatory diseases of the intestine impart a significant and negative impact on the health and well-being of human and non-human mammalian animals. Prolonged exposition to inflammation-linked effects can be the condition for the onset of inflammation-related pathologies; thus, understanding the underlying mechanisms of inflammatory diseases is mandatory to develop effective treatments and prevention strategies. Several are the intestinal pathologies which are closely related to the persistence of an inflammatory condition. Among them there is irritable bowel syndrome (IBS). IBS is a common pathological condition, being one of the most common functional gastrointestinal disorders (FGID). Studies have highlighted the persistence of mucosal inflammation at the microscopic and molecular level in IBS, with increased recruitment of enteroendocrine cells. Substantial overlaps between IBS and inflammatory bowel disease have also been reported [1]. The beneficial effects of nutraceutical compounds in human health have been emerging in the last decades. Nutraceuticals, such as herbal products or vitamins, are generally accepted as safer alternatives or supplementations to conventional therapy. An innovative product composed of L-Tryptophan, Pomegranate, and Inulin has been developed, thanks also to a formulation technology which allows the direct release of the active ingredients at the colon level. Since inflammation can be at the base of the aetiology of different intestinal pathologies such as IBS, in this work we want to evaluate the efficacy of the previously described blend of actives in contrasting an induced inflammatory condition. As inflammatory disease aetiologies are multifactorial, the use of appropriate animal models and associated metrics of disease are essential.
A prevention protocol using specific natural active ingredients will be evaluated in mice, using the dextran sodium sulphate (DSS)-induced intestinal inflammation [2] For the DSS protocol of experimental intestinal inflammation, 8-weeks-old C57Bl/6N mice (all females) have been used, as this mouse strain is highly susceptible to DSS treatment. through intrarectal injection. A total of 9 doses (1 dose/day from day 0 to day 9) have been administered for experimental groups (including vehicle, in which we administered only water), through intrarectal enema [3] Results showed that Compound significantly inhibited DSS-induced acute intestinal inflammation (Figure 1A-B), in terms of percentage of body weight loss, and reduced DAI, although with lower statistical significance when assessing DAI. No differences were observed between DSS and DSS + vehicle. Colon shortening is a parameter of intestinal inflammation. At day 10 mice were sacrificed and colon lengths were measured. Results confirmed the statistically significant anti-inflammatory effect of Compound, suggesting that these nutraceutical ingredients may prevent intestinal inflammation (Figure 1C). The anti-inflammatory effect of Compound in DSSinduced acute intestinal inflammation was confirmed histologically by a blinded pathologist, using RACHMILEWITZ score. According to RACHMILEWITZ score, results show that Compound was able to significantly inhibit experimental colon inflammation in comparison with control group (DSS + vehicle) in terms of flogosis and extension of flogosis (Figure 1D). Of note, in the DSSinduced model of acute intestinal inflammation, there is usually no development of fibrosis, and for this reason values in the graph are zero. Flogosis represent the presence of inflammatory infiltrate and edema, whereas ulceration represent epithelial damage [4]. This data suggests that nutraceutical ingredients in Compound may impact on recruitment of inflammatory cells (Figure 1E). Moreover, in DSS-induced mice (e.g., DSS and DSS + vehicle) layer stratification (mucosa, submucosa and musculari propria) is not maintained, a massive inflammatory infiltrate completely occupies both mucosa and submucosa, and crypt are severely damaged in most parts of the colon. The epithelium is eroded indicating the presence of ulcerations. Treatment with Compound significantly ameliorates experimental acute intestinal inflammation. We next determined the calprotectin [5] concentration in stool as a marker of mucosal inflammation representing leukocyte accumulation in stool (Figure 1F). At day 10, before sacrifice, stool have been collected and weighed to quantify Fecal calprotectin, a marker of mucosal inflammation representing leukocyte accumulation. Results showed that Compound was able to reduce Fecal calprotectin levels, expressed as mg/g of stool, but with no statistical significance, confirming the anti-inflammatory properties trend of Compound. The innovative product proposed has proven to be an effective defense in the treatment of intestinal inflammation also thanks to a formulation technology with direct release of the active ingredients into the colon, which helps to improve its effectiveness, reducing the possible side effects of these components and could hypothesize to place it for the treatment of irritable bowel syndrome (IBS).
Figure 1 Administration of compound significantly improves experimental acute intestinal inflammation.
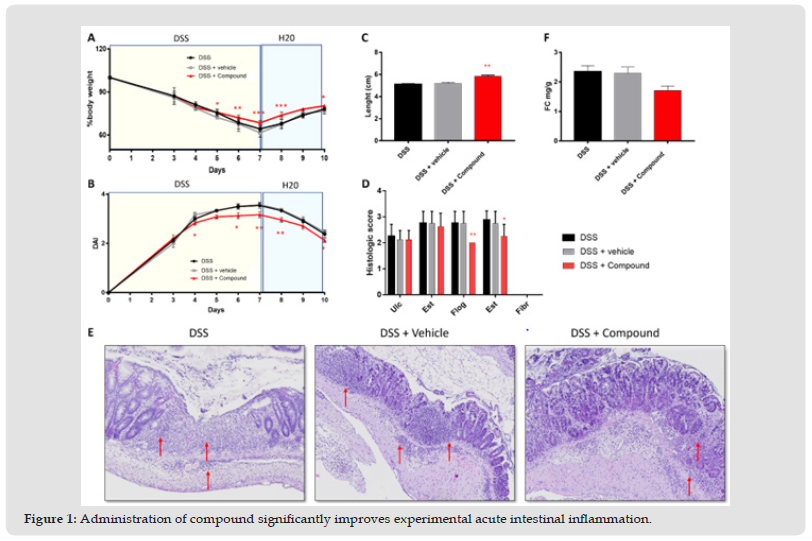
Percentage of body weight loss (A) and DAI (B) have been quantified at the indicated time points. Values mean ±SEM (n=8 per group). *P<0,05 vs DSS + vehicle; **P<0,01 vs DSS + vehicle by two-way ANOVA with multiple comparisons. (C) Administration of Compound significantly improves colon length reduction. Values are mean ±SEM (n=8 per group). **P<0,01 vs DSS + vehicle by one-way ANOVA with multiple comparisons. (D) Administration of Compound significantly reduces flogosis and extension of flogosis in DSS-induced mice. The graph represents histological grading of intestinal inflammation at day 10. (E) Compound reduces inflammatory infiltration in DSS-induced mice. Red arrows indicate inflammatory infiltrate. (F) Administration of Compound reduces fecal calprotectin levels in DSS-induced mice.
SDA has served as a speaker, consultant, and advisory board member for Schering Plough, Abbott (AbbVie) Laboratories, Merck and Co, UCB Pharma, Ferring, Cellerix, Millenium Takeda, Nycomed, Pharmacosmos, Actelion, Alfa Wasserman, Genentech, Grunenthal, Pfi zer, AstraZeneca, Novo Nordisk, Vifor, and Johnson and Johnson.
SM, SL and AV are Nutrilineasrl employees and have noconflict of interest to declare.


