Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Larisa Ivănescu* and Liviu Miron
Received: January 01, 2022; Published: January 06, 2023
*Corresponding author: Larisa Ivănescu, University of Life Science ‘Ion Ionescu de la Brad’, Al. Mihail Sadoveanu, no. 3, Iasi, 700490, Iași, Romania
DOI: 10.26717/BJSTR.2023.47.007575
Babesiosis is a tick-borne infectious disease caused by parasites of the genus Babesia which are intra-erythrocytic protozoan. Babesiosis is a disease with a worldwide distribution, causing mild to severe systemic clinical manifestations, characterized by erythrocyte destruction. In October and November 2022, two cases of babesiosis were presented at the Faculty of Veterinary Medicine in Iasi, Romania. A 14-year-old common breed dog and a 1-year-10-month-old dog, both cases presented clinical signs which suspected infection with Babesia sp., but upon examination of May-Grünwald Giemsa-stained peripheral blood smear and Diff Quick staining, the result was negative. Both cases came out positive in the WELL TEST Babesia canis-Babesia gibsoni AbCombo serological antibody detection test, indicating the presence of antibodies for both Babesia canis and Babesia gibsoni. We specify that none of the cases had been recently diagnosed with babesiosis and they had not received any specific treatment. We conclude that the diagnosis of babesiosis should include, in addition to the morphological examination under the microscope, the use of an antibody detection test, the sensitivity and specificity of which was shown to be high. The incubation period in babesiosis varies from 1 week to 2 weeks, during which the body can develop antibodies which can influence the diagnosis, considering that molecular biology techniques are not available to anyone.
Keywords: Blood Smear; Antibody Detection Test
Babesiosis is a vector-borne, tick-borne disease of worldwide distribution (Otranto, et al. [1]), caused by protozoa of the genus Babesia, infecting both domestic and wild animals (Uilenberg, et al. [2-5]). In the past, the diagnosis of canine babesiosis was made by microscopic morphological identification of the protozoan, establishing that the large forms were called Babesia canis, while Babesia gibsoni was used for the small forms (Solano-Gallego, et al. [6]). More recently, using molecular biology techniques, several species belonging to the Babesia genus were identified, such as: B. canis, B. vogeli, B. gibsoni, B. rossi, B. vulpes, B. conradae, B. negevi and an unnamed species of Babesia (Baneth, et al. [7-9]). The clinical picture in babesiosis is influenced by numerous factors, mainly by the infecting species, ranging from subclinical signs to severe forms including fever, lethargy, anorexia, pale mucous membranes and even septic shock (Koster, et al. [10]). The parasites complete their life cycle in red blood cells, where they replicate, initiating a process of cytotoxic destruction mediated by antibodies specific to circulating red blood cells (Zygner, et al. [11]). In addition, splenomegaly, haemoglobinuria, hypoxic lesions, lymphadenopathy and collapse associated with intra and extravascular haemolysis, thrombocytopenia, systemic inflammation, and pigmenturia are present (Irwin, et al. [5]). In more severe cases, damage to multiple internal organs, such as the kidneys, lungs, pancreas, and heart, were reported (Abalaka, et al. [12]). Canine babesiosis, also called «malignant jaundice», is a frequent and clinically significant disease (Penzhorn, et al. [13]).
The vector for B. canis canis is Dermacentor reticulatus in Europe (Barker, et al. [14]), the vector for B. canis vogeli is Rhipicephalus sanguineous in subtropical and tropical regions (LaVan, et al. [15]), and Haemaphysalis leachi is the vector for B. Canis Rossi in South Africa (Bashir, et al. [16]). B. canis vogeli generally produces a moderate infection, although deaths produced by it are frequently reported (Abalaka, et al. [12,17]). B. canis rossi infestation is frequently fatal in domestic dogs, in some cases even after treatment; while infestations with B. canis canis present an intermediate pathogenicity between B. canis vogeli and B. canis rossi (Uilenberg, et al. [2]). Babesia gibsoni, which is considered small babesia between 1.0 and 2.5 μm in size, was reported in infestation of dogs in North America, Asia, North and East Africa and Europe (Conrad, et al. [18]). Thus, canine babesiosis is characterised by a range of manifestations from subclinical to very severe life-threatening conditions, according to the immune response of the host and the species involved (De Tommasi, et al. [19]). In general, the diagnosis of canine infections with Babesia and the identification of the species was based on the morphology of the intraerythrocytic forms in the blood smear and the specificity of the host. Using the polymerase chain reaction (PCR), identification of piroplasms could be made with greater specificity and sensitivity than traditional methods (Jefferies, et al. [20]). Regarding the treatment of babesiosis, reports have shown that no drug has been considered sufficient so far for the treatment of babesiosis, especially B. gibsoni (Solano-Gallego, et al. [21]). Most of the drugs used do not prevent relapse and have side effects (Köster, et al. [22]).
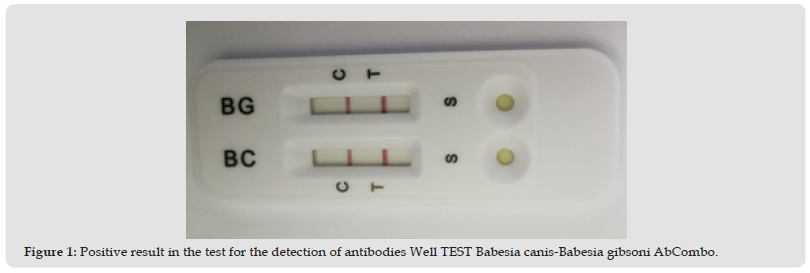
In November 2022, a C1 - 14-year-old male dog, common breed, presented himself at the Faculty of Veterinary Medicine in Iași, Romania. The patient had vomiting, jaundice, hypothermia, splenomegaly, gastroenteritis and refused food. Peripheral blood was smeared in thin layer, stained with May-Grünwald Giemsa and Diff Quick stain. The Rapid Test for veterinary use - WELL TEST Babesia canis-Babesia gibsoni AbCombo (BC-BG Ab) was also used. This is a rapid immunochromatographic test for the qualitative detection of Babesia canis antibodies (BC Ab) and Babesia gibsoni antibodies (BG Ab) in dog serum, blood or plasma. This test uses the lateral flow immunochromatographic method in double layer, sandwich format. The test is provided with a test window in order to see that the test is working and to read the result. The test window has an invisible area T (test) and a control area C (control). At the time of testing, the sample is placed in the test sample window. The mixture with the sample will migrate up the chromatographic membrane by capillary action and will react with the Babesia canis and Babesia gibsoni antigens on the membranes, in the area of the test line. If the sample contains antibodies to Babesia canis and Babesia gibsoni, a coloured line will appear in the test area, indicating a positive result. Used correctly, the test accurately indicates the presence of Babesia canis and Babesia gibsoni antibodies in the sample.
The second case C2 was a 1-year and 10-month-old dog, common breed, 40 kg.
The reason for the presentation at the Faculty of Veterinary Medicine was the presence of a fever of 39.8°C, apathy, loss of appetite, slightly jaundiced pale mucous membranes. May- Grünwald Giemsa-stained peripheral blood smear and Diff Quick staining for the detection of intraerythrocytic parasites were negative. The rapid antibody detection test was also used.
Following blood tests in patient C1:
VSH: (N˂10mm/h); mm/2h (N˂20)
Reticulocytes (Crezyl blue vital staining): 36.6% (N˂1.5) x 10³/ μl (N˂100).
The cytological examination was done from blood on EDTA; blood smear was stained using May-Grünwald Giemsa and Diff Quick staining:
A. Anisocytosis, macrocytes, En 4.4%, Jolly bodies, stomatocytes, erythrocyte aggregates, annulocytes.
B. Reactive Lf, total Nf 83% (35x10³/μl), young Nf (1-2 lobes) 64.8%, Nf (3 lobes) 29.6%, Nf (4-5 lobes) 5.6%, Eo 3.1% (1.30x10³/μl), Mo 8.5% (3.59x10³/μl), Lf 5.4% (2.28x10³/μl).
C. Platelet aggregates.
Cytological and haematological diagnosis (reference values: The Merck Veterinary Manual, ed. 8):
1. Normocytic, normochromic, hyper-regenerative anaemia;
2. Leucocytosis;
3. Left deviation of the neutrophilic nuclear index;
4. Relative lymphopenia;
5. Monocytosis.
No intraerythrocytic annular formations, associated with Babesia sp. parasites, were detected during the blood smear analysis. In both cases, the peripheral blood smear analysis was negative, and the rapid antibody test was positive, detecting the presence of antibodies to Babesia canis and Babesia gibsoni (Figure1). The treatment in both cases was done with the administration of Imidocarb 6mg/kg with repetition after 14 days; combining clindamycin 30mg/kg 2 times/day for 30 days. RX Hepato Support for 60 days and Bioprotect for 30 days. The condition of the patients improved starting from the third day after the treatment.
Figure 1 Positive result in the test for the detection of antibodies Well TEST Babesia canis-Babesia gibsoni AbCombo.
The pathogenicity of Babesia microorganisms is primarily given by the species and the strain involved. Host factors such as the age and immunological response of the host also play an important role. In the beginning, babesia multiply and invade a large number of red blood cells, producing severe anaemia, which can also be maintained by the presence of anti-erythrocytic autoantibodies and immune complexes (which can also produce glomerulonephritis), producing red blood cell lysis by complement. The fragility of erythrocytes increases, liver and kidney dysfunctions occur, generating jaundice and albuminuria with haemoglobinuria. Hypoxia produced by disseminated intravascular coagulation occurs, followed by anoxia. The agglutinated parasitized red blood cells cause the blockage of capillary circulation, leading to the appearance of nervous signs, with perivascular oedema in the brain. The incubation period for all piroplasms is about 1 week. However, the period can be shorter (2–3 days) or longer (10–15 days), according to the immune status of the host. IgG antibodies usually appear 2-3 weeks after infection (Vercammen, et al. [23]).
The classic (acute) form is the most frequent (> 50% of the cases), the chronic form includes different clinical manifestations: either a relapse with the appearance of the clinical picture 15 days after the first diagnosis, as a result of a deficiency of the immune system or as a result of emergence of parasite chemoresistance. Thus, an antibody detection test can be used all the time, in addition to the classic blood smear, being easy to use and within reach of the medical offices, without requiring special equipment, as in the case of the PCR technique. The PCR technique has the highest specificity and sensitivity in the diagnosis of acute babesiosis, however, in chronic babesiosis, molecular biology methods can give a false negative result. The cases presented underline the complexity of the pathogenicity of the parasite Babesia sp. and the very different response of each individual organism. In addition, the high sensitivity and specificity of the Well TEST Babesia canis- Babesia gibsoni AbCombo test was shown, detecting the antibodies in the acute period of the disease, neither of the cases having been previously treated for babesiosis, therefore we cannot state that these cases are relapses.
We conclude that the use of the Well Test Babesia canis-Babesia gibsoni AbCombo antibody test in veterinary clinics can be of real use in the correct diagnosis of babesiosis in dogs. The current study shows that the Well Test Babesia canis-Babesia gibsoni AbCombo antibody test can also be used in acute babesiosis, having a high specificity and sensitivity.


