Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Leta Melaku*
Received: December 16, 2022; Published: January 05, 2023
*Corresponding author: Leta Melaku, Assistant Professor of Medical Physiology, Department of Biomedical Sciences, College of Health Sciences, Arsi University, Ethiopia
DOI: 10.26717/BJSTR.2023.47.007570
Background: Potassium (K+) ion channel activity is an important determinant of
vascular tone by regulating cell membrane potential (MP). This study aims to describe
the effect of hypertension on the physiological function of major potassium channels
within blood vessels.
Method: Studies were accessed through an electronic web-based search strategy
from PubMed, Cochrane Library, Google Scholar, Embase, PsycINFO, and CINAHL by
using a combination of search terms.
Results/Discussion: Each of K+ channels (Voltage-dependent K+ (Kv), large-conductance
Ca2+-activated K+ (BKCa), and ATP-sensitive K+ (KATP) channels) is responsive
to a number of vasoconstrictors and vasodilators, which act through protein kinase
C (PKC) and protein kinase A (PKA), respectively. Impaired Kv, KATP, and Kir
channel function have been linked to a number of pathological conditions, like hypertension.
Vasoconstriction and the compromised ability of an artery to dilate are likely
consequences of defective K+ channel function in blood vessels during these disease
states. In some instances, increased K+ channel function may help to compensate for
increased vascular tone. Endothelial cell dysfunction is commonly associated with
cardiovascular disease, and altered activity of nitric oxide, prostacyclin, and endothelium-
derived hyperpolarizing factors could also contribute to changes in resting K+
channel activity, Em, and K+ channel–mediated vasodilatation.
Conclusion/Perspectives: Potassium channels importantly contribute to the
regulation of vascular smooth muscle (VSM) contraction and growth. Activation of
K+ channels leads to membrane hyperpolarization and subsequently vasodilatation,
while inhibition of the channels causes membrane depolarization and then vasoconstriction.
Keywords: Arterial Vascular Smooth Muscle Cells; Potassium Channels; Chronic Hypertension
Abbreviation:SHRs: Spontaneously Hypertensive Rats; VSMC: Vascular Smooth Muscle Cell; VOCC: Voltage-Operated Calcium Channel; Popen: Open Probability; NO: Nitric Oxide; CO: Carbon Monoxide; WKYs: Wistar-Kyoto Rats
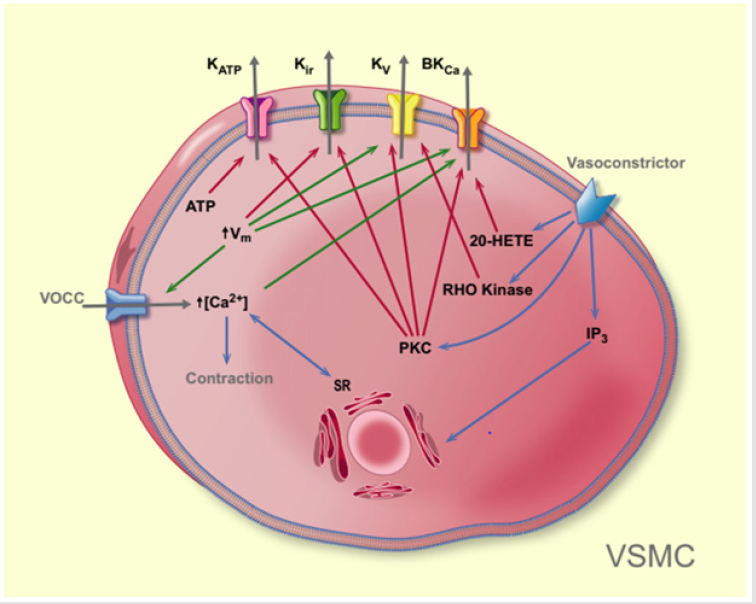
Potassium channels are the dominant ion conductive pathways in vascular muscle cells. As such, their activity importantly contributes to the determination and regulation of membrane potential and vascular tone [1,2]. To date, four distinct types of K+ channels have been identified in vascular smooth muscle (Figure 1). The following depolarization and increase in [Ca2+]i can activate K+ chan nels to buffer the vasoconstriction (shown with green arrows). See text for details. Blue arrow = effect; red arrow = deactivation; green arrow = activation. VOCC: voltage-activated calcium channel, Vm: membrane potential, SR: sarcoplasmatic reticulum, PKC: protein kinase C, IP3: inositol trisphosphate, 20-HETE: 20-hydroxyeicosatetraenoic acid.
Figure 1.Pathways leading to vasoconstriction via deactivation of K+ channels in vascular smooth muscle cells (shown with red arrows).
Voltage-Dependent K+ Channels (KV Channels)
KV channels consist of four α-subunits, each subunit is associated with an additional β-subunit influencing the characteristics of the channel. Conductances between ~ 4 and 70 pS have been reported for single KV channels in vascular smooth muscle cells depending on preparation and experimental conditions [3,4]. Compared with the process of activation, KV channel inactivation is relatively slow and involves an initial peak in the KV current [5]. The various constituents of the KV current have been identified and the KV channels present in vascular smooth muscle have been divided into groups based on their voltage dependence and pharmacological data. KV channels exhibit strong voltage dependence. Cell membrane depolarization leads to activation of KV channels and an increased hyperpolarizing outward K+ current. The resulting hyperpolarization of the vascular smooth muscle cell (VSMC) inactivates voltage-operated calcium channel (VOCC) channels and consequently decreases VSMC tone (Figure 2) (1,6). The threshold for activation of KV channels is approximately -50 mV. Sustained depolarization leads to inactivation of KV channels. Inactivation is slower than activation, and the steady-state current via these channels is determined by the balance between these processes. Thus, the open probability (Popen) of these channels is a product of the probability that they are available (not inactivated) and the probability that they are activated [7,8]. KV channels are also activated by the cAMP/PKA pathway and inactivated by PKC as well as by a decreased pH. Furthermore, Rho kinases have been reported to suppress KV currents in rat cerebral arteries [9].
Figure 2.Pathways leading to vasodilation via activation of K+ channels in endothelial cells or vascular smooth muscle.
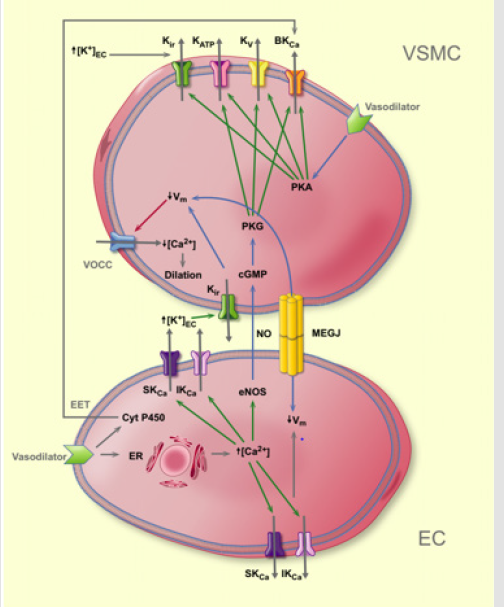
Function of vascular KV channels: Small-scale depolarization in vascular smooth muscle cells leads to an influx of Ca2+ through L-type Ca2+ channels and activation of the contractile machinery. KV channels, also called the delayed rectifier, may contribute to the regulation of the resting membrane potential and thus the tone of VSMC (Figure 2) [10,11]. It is suggested that the physiological role of KV channels is to buffer membrane depolarization to maintain resting vascular tone [1]. The presence of KV channels in EC has been indicated, but their precise role in the control of EC function has yet to be clarified.
ATP-Sensitive K+ Channels (KATP Channels)
KATP channels are hetero-octameric complexes which consist of eight protein subunits; four α-subunits from the Kir family (Kir6.1 or Kir6.2) forming the pore and four regulatory subunits which belong to the family of sulfonylurea receptors (SUR1, SUR2A, and SUR2B) [12]. The molecular diversity that exists between species and tissues in terms of their KATP channels is magnified by the presence of multiple isoforms of SUR; however, they clearly fall into two distinct categories: small/medium conductance and large conductance [13]. Values between 7 and 15 pS have been reported for small/ medium-conductance KATP channels at 6 mM [K+]o, whereas values between 20 and 25 pS have been reported at 60 mM [K+]o; conductances in the range of 20 ~ 50 pS have been identified in symmetric high K+ in arterial smooth muscle cells [14]. The clinical implications of the inhibition of KATP by sulfonylureas relate to their use as oral agents in the therapy of maturity-onset diabetes mellitus [15]. KATP channels in smooth muscle are known to be inhibited by antidiabetic sulphonyl urea drugs, such as glibenclamide and tolbutamide. Glibenclamide is the most frequently used inhibitor of KATP channels in studies of arterial smooth muscle, with a half-inhibition value between 20 and 200 nM [16]. Tolbutamide, with a half-inhibition value of 350 μM in smooth muscle, is considerably less effective than glibenclamide as a hypoglycemic agent [17]. 4-morpholinecarboximidine- N-1-adamantyl-N’-cyclohexyl hydrochloride (U-37883A) and 5-hydroxydecanoate are selective inhibitors of nonvascular KATP channels with half inhibition values of 0.26 μM and 0.16 μM, respectively [18]. External Ba2+ also could be acting as an inhibitor of KATP channels in smooth muscle, with a half-inhibition value of 100 μM at -80 mV [19]. The KATP channels are not affected by iberiotoxin and are less sensitive to tetraethylammonium (TEA) [20]. The vasodilation induced by synthetic K+ channel activators, including cromakalim, levcromakalim, nicorandil, pinacidil, minoxidil, diazoxide, and BRL-55834 (a novel potassium channel activator), can be successfully blocked by glibenclamide; thus, they are believed to hyperpolarize the cells in vascular smooth muscle by targeting KATP channels [13]. However, other K+ channel blockers, such as iberiotoxin, apamin, or low concentrations of TEA are ineffective on KATP channels [21].
Function of vascular KATP channels: It has been shown both in vitro and in vivo that a block in KATP channels leads to vasoconstriction and membrane depolarization in various types of vascular smooth muscle (Figure 2) [22]. KATP channel activation is closely associated with several pathophysiological responses, including systemic arterial dilation during hypoxia, reactive hyperemia in the coronary and cerebral circulation, and acidosis and endotoxic shock-induced vasodilation. Moreover, the inhibition of KATP channels leads to impaired coronary and cerebral autoregulation [23]. KATP channels could be controlled by cell metabolism via the concentration of ATP and/or the ATP/ADP ratio, and it is possible that they have a role in the adaptation of vascular tone to the metabolic needs and partial pressure of oxygen (PO2) of the tissue [24]. Furthermore, since the channels are inhibited by PKC, there is a potential for mediation of the effect of vasoconstrictors like norepinephrine (NE) and ANG II [11]. Outward currents from these channels are stimulated by PKA and/or PKG, and it is suggested in some studies that this pathway mediates the vasodilatory action of adenosine [3]. ECs also expresses KATP channels and may participate in shear stress- and hyperosmolarity-mediated vasodilation [11].
Inward Rectifier K+ Channels (Kir Channels)
The Kir channels are tetramers where each α-subunit has two transmembrane domains. Six families of Kir channels are known (Kir1– 6), where Kir2 is the classical inward rectifier channel and Kir6 is the ATP-sensitive channel [25]. A unique feature of Kir channels is the small increase (~5–12 mM) in [K+]o increasing the outward current (Figure 2). This mechanism is mediated by the interaction of K+ with polyamines and/or Mg2+ in the channel pore [26]. An increase in the single-channel conductance has recently been suggested to also contribute to the increased outward current [27]. The Nernst equation predicts that increased [K+]o leads to a less negative EK, and thus a depolarization of the cell membrane is to be expected, but as the small elevation in [K+]o also increases the outward K+ current, the net effect is a hyperpolarization of the cell membrane. If [K+]o is increased further (more than ~20 mM), the depolarizing effect dominates [28,29]. The single-channel conductance of the Kir channels is reported to be ~20 pS [3]. They are coined “inward rectifier” channels because of their propensity for carrying current in the inward direction. Only a few papers have addressed the single-channel properties of Kir channels in vascular smooth muscle [30]. Ba2+ is a general blocker of K+ channels, but at concentrations <100μM it is considered specific for Kir channels with a reported half-maximal inhibition concentration of 2.2 μM at -60 mV. However, Ba2+ is much less effective on KATP channels (Kd, 200 μM at –60 mV), BKCa channels (Kd>10 mM), and KV channels (Kd >1 mM). Therefore, at concentrations below 50 μM, Ba2+ is a selective blocker of Kir channels in vascular smooth muscle. Cs+, which acts from outside the cell, also inhibits Kir currents in vascular smooth muscle with a Kd of 2.9 mM at –60 mV (13). External Ca2+ and Mg2+ partially block Kir channels. For example, at –60 mV, 5 mM Ca2+ or Mg2+ reduced the Kir current by 47% and 41%, respectively.
Function of vascular Kir channels: Kir channels are found predominantly in VSMC from microvessels, and the main isoform found is Kir 2.1. However, Wu et al. suggested that the Kir channels in cerebral arteries contain both Kir2.1 and Kir2.2 [31]. In addition, Kir2.1 and Kir2.4 expression has been detected in cultured human pulmonary arterial smooth muscle cells [30]. The Kir channels in vascular smooth muscle cells mediate inward currents at membrane potentials that are negative relative to the EK and small outward currents at membrane potentials that are positive relative to the EK [32]. Given that the membrane potential of vascular smooth muscle is normally positive relative to the equilibrium potential (EK), a physiological role for the inward rectifier channel requires an outward current through the channel (Figure 2) [33]. Inward rectifier K+ channels are abundant in the smooth muscle of small-diameter resistance vessels [34]. Although the exact function of Kir channels in vascular smooth muscle is still incomplete, there are two basic possibilities. First, Kir channels contribute to the resting membrane potential and resting tone in small-diameter vascular smooth muscle [35]. Second, Kir channel activation in response to moderate increases in the extracellular K+ concentration (up to 10–15 mM) may cause vasodilation [36,37]. In accord with the sensitivity to [K+]o described above, Kir channels might participate in K+ induced vasodilation described in the metabolic regulation of blood flow and in the endothelium-derived hyperpolarizing factor (EDHF) response elicited by K+ release via small-conductance (SKCa) and intermediate- conductance (IKCa) calcium channels. It is also possible that part of the K+ induced hyperpolarization/vasodilation is mediated by activation of Na+-K+ ATPase. In addition to VSMC, Kir channels are found in endothelial cells where Kir 2.1 is supposed to be the main isoform [38].
Ca2+-Activated K+ Channels (BKCa Channels)
Similar to KV channels, BKCa channels are comprised of a pore-forming α-subunit and a regulatory β-subunit. The α-subunits contain six transmembrane-spanning domains (S1–S6), including a voltage sensor (S4), which form the pore. Furthermore, the α-subunits produced from a gene slo contain an additional seventh transmembrane region (S0) at the exoplasmic NH2 terminus [39]. There are also four β-subunit isoforms (β1-4), each with two transmembrane domains, which may be associated with the α-subunits in a 1:1 ratio [40]. Of the four isoforms, the β1 subunit is the predominant isoform in vascular smooth muscle. The major function of the β-subunits is to enhance the Ca2+ sensitivity of the channel [41,42]. BKCa channel is also known as KCa2+ (Slo) and their conductance is between 200 –250 pS [43]. These channels are voltage sensitive, and the voltage sensitivity is modulated by [Ca2+]I (Figure 2). BKCa channels are activated by changes in the intracellular Ca2+ concentration and membrane depolarization [41,44]. The efflux of K+ that results from BKCa channel activation can be used to counteract pressure- or chemical-induced depolarization and vasoconstriction. They are also activated by protein kinases including cAMPand cGMP-dependent protein kinases (PKA and PKG) [45,46]. Most studies indicate that PKC inhibits BKCa channels, but one study has shown that PKC via cGMP activates BKCa. As nitric oxide (NO) stimulates the cGMP/PKG pathway, activation of nitric oxide (NO) synthase might lead to increased BKCa activity [47]. Moreover, carbon monoxide (CO) has been reported to activate BKCa channels via a PKG dependent mechanism or by binding to a heme group on the channel. Pharmacologically, BKCa channels may be blocked by external TEA, iberiotoxin, and charybdotoxin at half inhibition values of 200 μM, < 10 nM, and ~ 10 nM, respectively [48,49]. Of these blockers, iberiotoxin is the most selective blocker; it has a very low half-inhibition value and its value is ineffective on other types of K+ channels [40]. Charybdotoxin has been used as a selective BKCa channel blocker; however, it also affects KV channels as well as intermediate conductance Ca2+-activated K+ channels [41]. BKCa channels are not affected by glibenclamide, Ba2+, or apamin, which blocks low-conductance Ca2+-activated K+ channels at their working concentrations [1]. Both NS-004 (an activator of Ca2+-dependent K+ Channels) and NS-1619 (a selective large conductance Ca2+-activated K+-channel activator) have been used to stimulate BKCa channels in smooth muscle, but they are of limited value given their nonspecific inhibitory effects on L-type Ca2+ channels [50].
Function of vascular BKCa: At negative Vm, activation requires [Ca2+]i in the range of 1–10μM, which exceeds that found in the bulk cytoplasm under resting conditions. It is, therefore, controversial whether BKCa channels are active at resting Vm and VSMC [Ca2+] i (Figure 2). However, BKCa channels have an important function by buffering vasoconstrictor responses [1]. The increase in VSMC [Ca2+]i, resulting from agonist stimulation or activation of the myogenic mechanism, activates BKCa channels, attenuates depolarization, and opposes vasoconstriction [51]. The increase in [Ca2+]i necessary for activating BKCa need not be global, but may be restricted to so-called microdomains within the cell. Ca2+ recruitment via entry and release from intracellular stores may cause localized elevations in [Ca2+]i, so-called Ca2+ sparks, able to activate BKCa channels. Recent evidence shows that BKCa channels and voltage-operated calcium channels (VOCC) form complexes in the cell membrane, which could provide an efficient mechanism for attaining local Ca2+ concentrations of a magnitude necessary to activate BKCa channels [52]. In isolated coronary and cerebral arterial myocytes from rabbits, it is shown that BKCa channels open simultaneously with the L-type Ca2+ channels [53]. In addition, Ca2+ released via ryanodine- sensitive Ca2+ channels in the sarcoplasmic reticulum attenuates vasoconstriction via activation of BKCa channels. It is reported that vasoconstrictors could exert their effect by PKC-mediated inhibition of BKCa channels [54].
Blue arrow = effect; red arrow = inhibition; green arrow = activation. ER: endoplasmic reticulum, Cyt P450: cytochrome P450, NOS: nitric oxide synthase, Vm: membrane potential, EET: epoxyeicosatrienoic acids, MEGJ: myoendothelial gap junctions, K+ +:KEC released from the endothelial cell, cGMP: cyclic GMP, PKG: protein kinase G, VOCC: voltage-activated calcium channel, PKA: protein kinase A.
PubMed, Cochrane Library, Google Scholar, CINAHL, Embase, and PsycINFO database were used for studies reporting the molecular and cellular emerging role of arterial vascular smooth muscle cells potassium channels in chronic hypertension from study conception to May 2021. Zotero reference management software for Windows was used to download, organize, review and cite the articles. I also manually searched cross-references in order to identify additional relevant articles. A comprehensive search was performed using the following search terms: “role of potassium channels”, “pathobiology of hypertension”, and “molecular and cellular emerging role potassium channels in chronic hypertension”. Boolean operators like “AND” and “OR” were used to combine search terms.
BKCa Channels in Hypertension
There is strong evidence that the functional role of BKCa channels is enhanced in vascular muscle during chronic hypertension in vessels from various anatomic regions. Increases in both Ca2+ influx and BKCa channel activity can be detected even in prehypertensive spontaneously hypertensive rats (SHRs) [55], so genetic factors may play at least some role in these changes. Increased BKCa channel function may be also a consequence of elevated blood pressure, as it can be induced over several weeks by surgical or pharmacological interventions that cause hypertension [56], and it can be reversed by antihypertensive therapy [57]. Therefore, BKCa channel function may be increased in arterial smooth muscle cells as a protective mechanism against progressive increases in blood pressure and may provide a negative feedback mechanism that helps to restrict the increased pressure and vascular tone. Such a mechanism would therefore act to limit pressure-induced vasoconstriction and to preserve local blood flow. Electrophysiological measurements obtained under voltage-clamp conditions in arterial myocytes isolated from hypertensive animals have confirmed that the whole-cell K+ current through BKCa channels is enhanced in comparison with currents recorded from normotensive myocytes [56,58]. Another study has reported that BKCa channel open probability may be increased in the renal vasculature owing to higher intracellular Ca2+ levels during hypertension. Thus, activity of the BKCa channel appears to be greater in hypertensive arteries, and it may be the primary determinant of resting K+ efflux and hence, vascular muscle Em in hypertension [59].
KV Channels in Hypertension
Unlike BKCa channels, there is a decreased function of arterial KV channels in hypertension given that vascular Em appears to be more depolarized and the activity of KV channels, like BKCa channels, is voltage dependent [60]. However, a depolarized Em and a higher intracellular Ca2+ level in myocytes from chronically hypertensive rats may represent a detrimental positive feedback mechanism resulting in decreased vascular KV channel activity. Reduced KV channel activity could therefore be an important factor underlying the depolarized Em and enhanced myogenic vascular tone reported during chronic hypertension [61,62]. Paracrine also influences potentially underlying altered K+ channel activity in hypertension have not yet been extensively explored and could be numerous. Parathyroid hypertensive factor is thought to increase Ca2+ influx into vascular muscle cells via L-type, voltage-activated Ca2+ channels (probably due to KV channel inhibition) and consequently to enhance vascular responses to depolarizing and constrictor stimuli [63,64]. In an analogous manner, decreased KV channel function in the pulmonary circulation is reported to cause depolarization and vasoconstriction in patients with primary pulmonary hypertension. Because NO may activate KV channels in some arteries [65], impaired bioavailability of endothelium-derived NO in hypertension could lead to vascular depolarization and contraction that are partly due to inhibition or closure of KV channels. Accordingly, increased knowledge of mediators that modulate KV channel function could provide important insight into the mechanisms of increased vascular tone during chronic hypertension and may reveal novel targets for pharmacological prevention of vascular dysfunction.
KATP Channels in Hypertension
Several studies suggest that the function of vascular KATP channels is impaired during hypertension. The vasodilator response associated with cerebral blood flow autoregulation during systemic hypotension, which is mediated by KATP channel activation, is also impaired in chronically hypertensive rats [66,67]. These findings suggest that KATP channel dysfunction may also interfere with vascular responsiveness to endogenous vasodilator stimuli. Impaired KATP channel–mediated vascular effects may not necessarily occur for all endogenous KATP channel activators, however, because responses to some vasodilators that increase intracellular cAMP levels (ie, forskolin and norepinephrine) are preserved in the basilar artery of hypertensive rats [68]. Thus, a defect in a particular aspect of KATP channel function could account for the abnormal responses during hypertension. Moreover, increased effects of angiotensin II and protein kinase C are associated with various forms of chronic hypertension, and both mediators may inhibit KATP channel function in vascular smooth muscle. Importantly, impairment of KATP channel– mediated vascular responses can be restored to near-normal levels by long-term treatment of high blood pressure [67,69]. These findings emphasize the importance of antihypertensive therapy for correcting many abnormalities in vascular smooth muscle function. However, there is very few information available currently on the effects of hypertension on KATP channel function in human blood vessels. It will be especially important to clarify whether this phenomenon occurs in humans; if it is restricted to certain vascular beds, this knowledge may then be critical in predicting the viability of using KATP channel openers as a potential new class of antihypertensive therapy.
Kir Channels in Hypertension
.There is indirect evidence that vascular Kir channel function may be altered during chronic hypertension. Earlier studies reported that K+-induced vascular relaxation was either augmented or impaired in several models of hypertension. McCarron and Halpern [70] also reported that Ba2+-sensitive vasodilator responses to >7 mmol/L K+ were impaired in posterior cerebral arteries isolated from stroke-pronespontaneously hypertensive rats (SHRs) in comparison with vessels from Wistar-Kyoto rats (WKYs), perhaps suggesting impaired Kir channel function in the cerebral circulation during hypertension. Thus, it is possible that chronic hypertension leads to decreased expression and/or function of vascular Kir channels and expression of a compensatory vasodilator mechanism(s) that is upregulated.
Vascular tone plays an important role in the regulation of blood pressure and the distribution of blood flow between and within the tissues and organs of the body. Current evidence indicates that vascular smooth muscle cells express at least 4 different types of K+ channels (i.e., BKCa, Kir, KV, and KATP channels). Increased appreciation of the diversity of vascular cell types present throughout the circulation and the relevance of specific K+ channel functions in different cell types. As further progress is made, particularly through molecular and electrophysiological approaches, in unravelling the relationship between K+ channel structure and function, we will obtain a clearer picture of the ways in which vascular smooth muscle cell K+ channels are affected by different pathophysiological conditions like hypertension. With the development of more selective pharmacological modulators, activation of vascular K+ channels would seem to be a very promising direction for therapy in numerous vascular disease states associated with vascular constriction and depolarization.
Ethics Approval and Consent to Participate:Not applicable.
Consent for Publication:Not applicable.
Availability of Data and Material:The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests:The authors declare that there is no conflict of interests regarding the publication of this paper.
Funding:Nil support in financial or other manner
Authors’ Contributions:LM had participated in the design of the study, data analyses, and manuscript preparation; and the authors could have read and approved the final manuscript.
Acknowledgements:The Author is grateful to College of Health Sciences Research and Community Office of Arsi University as well as researchers who their documents were used in the preparation of the review.


