Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Essam Eldin Abdelhady Salama*, Sanna Alshaarawy, Khaleel Ibrahim Alyahya, Tahani Ahmad AlMatrafi and Waad Mohammed Assiri
Received: December 08, 2022; Published: January 04, 2023
*Corresponding author: Essam Eldin Abdelhady Salama, Department of Anatomy and Embryology King Saud University, Riyadh, Kingdom of Saudi Arabia
DOI: 10.26717/BJSTR.2023.47.007566
Maternal immunization is important to protect the newborn; a protective level of Ig G. must be present in the mother’s blood at a time when it can be transferred to the fetus through feto-maternal interface. The aim of the study was to evaluate uptake of the immunoglobulin G (Ig G.) during pregnancy, across the endometrium by ultrastructure immunocytochemistry. Thirty pregnant female rats were used, they were divided into three groups; 1st week of gestation (group 1), 2nd week of gestation (group 2), and 3rd week of gestation (group 3), the endometrium of the pregnant females were examined by gold conjugate ultrastructure immunocytochemistry. During stages of gestation gold particles labelled (Ig G.) were detected in uterine lumen, luminal epithelium, and, in the sub-epithelial stroma, and also in lumens and stroma of the blood vessels. The results: (group1); the gold particles labelling (Ig G.) were seen in the uterine lumen, in the apical cytoplasm and the uterine surface of the plasma membrane. (group 2); a moderate amount of gold particles labelling (Ig G.) was detected in the luminal epithelium, within vesicles, and in multivesicular bodies. (group 3); a significant amount of gold particles labelling (Ig G.) were seen in the uterine lumen and in the apical epithelium, as well as in the in the sub-epithelial stroma, including blood vessels stroma and lumen.
Keywords: Immunization; IgG; Gold Labeling; Endometrium; Pregnancy; Immunocytochemistry
Anti-infectious fetal protection is provided by several factors acting together, the uterine cavity contains innate immune detection and effector systems that maintain sterility [1]. Maternal antibodies transported across the placenta protect the newborn, the placental transfer of maternal immunoglobulins to the fetus is a specific adaptive mechanism which minimizes the deficiencies in antibody production and donates short-term passive immunity [2]. The substances that pass from maternal blood to fetal blood must traverse the histological barrier; that include, the multinucleated syncytiotrophoblasts, the endothelial cells of the fetal capillaries, and, the villous stroma [3]. The histological barrier separates the blood in maternal and fetal circulation, it is not a simple physical barrier, a wide range of substances, are efficiently transferred actively or passively through the placenta to the fetus, which is essential for normal fetal growth, immunity, and, development. Substances of very high molecular weight do not usually traverse the placenta, but there are a few exceptions, such as immunoglobulin G (Ig G.), which has a significant amount transferred across the placenta [4]. Transported Ig G. crosses the syncytiotrophoblast covering the chorionic villi, and the endothelium of fetal capillaries within the villi, these cell layers are separated by their basal laminas, and in some regions by the stromal connective tissue [5]. According to Bright, et al. [6], Ig G. crosses the fetal vessel endothelium by a transcellular route, if maternal immunization is to protect the newborn, a protective level of specific Ig G. must be present in the mother’s blood at a time when it can be transferred to the fetus.
The placental transfer of maternal immunoglobulins to the fetus is a specific adaptive mechanism that to some extent, minimizes the deficiencies in antibody production and permits short-term passive immunity [2].The time period in which maternal immunization is effective depends on the timing of the mother’s immune response to the vaccine, and also the timing of maternal–fetal Ig G. transport, the gestational age[7]. As gestation progresses, the syncytiotrophoblast increases the surface area for Ig G. uptake from maternal blood, and also allow access to the fetal vessel endothelium [3]. Ig G. transfer from mother to fetus begins as early as 13 weeks of gestation, and transport happens in a linear fashion as the pregnancy progresses, with the largest amount transferred in the third trimester, [8]. According to Okoko, et al. [9] expression of Ig G. receptor is dependent on gestational age; a reduced placental transfer of antibodies is observed at early stages of gestation, and seems to be more highly expressed in the third trimester of human pregnancy.
Thirty adult female and ten male Wistar rats (Rattus norvergicus), three months old, weighing 200 to 300 g were used. The animals were housed in standard cages, in a controlled temperature room (22ºC), with a 12 h light: 12 h dark cycle, food and tap water were available ad libitum. According to Archunan and Dominic, [10], daily vaginal lavage was done to identify the preestrus period in the female rats, once an animal showed positive results it was caged overnight with breeding males, a second lavage was done to determine sperms, which was designated as the 1st day of gestation. The pregnant female rats were divided into three groups each one contains 10 rats, they were placed individually in a plastic cage. The animals were sacrificed at the end of the 1st week of gestation (group 1), the end of the 2nd week of gestation (group 2), and the end of the 3rd week of gestation (group 3). After scarification, the uterine horns were perfused with 4% paraformaldehyde and 0.1 glutaraldehyde in 0.1 M phosphate buffer for fixation, the endometrium was washed after fixation in sucrose buffer and dehydrated in ascending grades of ethanol. the capsules were infiltrated at low temperature and then transferred to wire capsule holder for ultraviolet polymerization of monomeric Lowicryl. Ultrathin section was cut and mounted on mesh copper grids immunoglobulin was done according to Polak, et al. [11].
First Week Gestation: (Group 1)
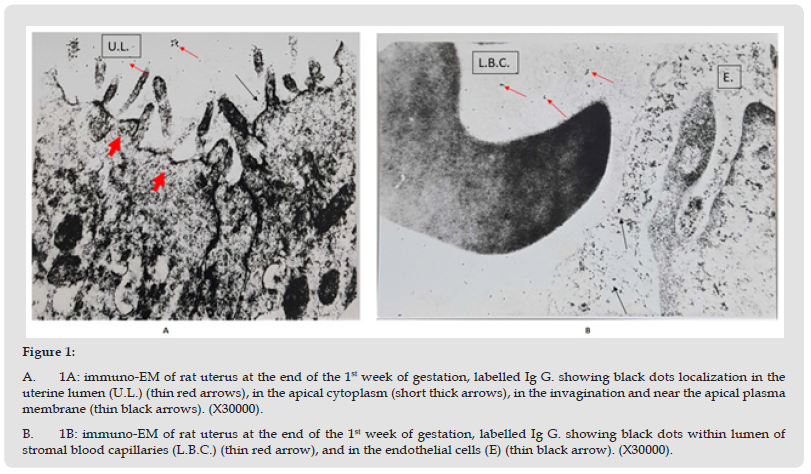
There was definite labelling of Ig G. in the endometrium indicated by the presence of gold particles, as black dots; few gold particles were seen to be localized in the uterine lumen. In the luminal epithelium the gold particles were demonstrated in the apical part of the cells, bound to the membranes of the invigilated pits, at the base of the microvilli, in the apical vacuoles, and, close to the apical part of the lateral plasma membrane (Figure 1-A). The endometrial stroma showed black dots of gold particles, demonstrated in the connective tissue close to the blood capillaries, in addition, gold particles were seen freely within the lumen of stromal blood vessels (Figure 1-B).
Figure 1 A. 1A: immuno-EM of rat uterus at the end of the 1st week of gestation, labelled Ig G. showing black dots localization in the uterine lumen (U.L.) (thin red arrows), in the apical cytoplasm (short thick arrows), in the invagination and near the apical plasma membrane (thin black arrows). (X30000). B. 1B: immuno-EM of rat uterus at the end of the 1st week of gestation, labelled Ig G. showing black dots within lumen of stromal blood capillaries (L.B.C.) (thin red arrow), and in the endothelial cells (E) (thin black arrow). (X30000).
Second Week of Gestation: (Group 2)
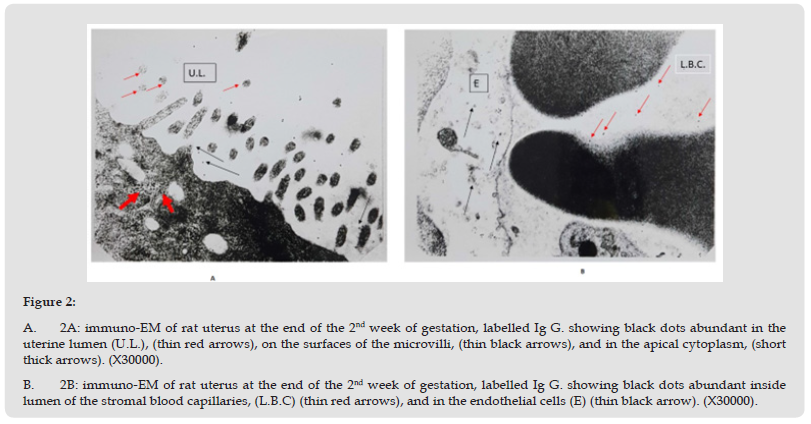
The gold particles as black dots were seen in both the luminal epithelium and the endometrial stroma. The uterine lumen and luminal epithelium showed moderate concentration of the gold particles, in the apical cytoplasm, the microvilli showed few gold particles on their surface, (Figure 2-A). The gold particles were also identified in moderate amount in the endometrial stroma and in the stromal blood capillaries, (Figure 2-B).
Figure 2 A. 2A: immuno-EM of rat uterus at the end of the 2nd week of gestation, labelled Ig G. showing black dots abundant in the uterine lumen (U.L.), (thin red arrows), on the surfaces of the microvilli, (thin black arrows), and in the apical cytoplasm, (short thick arrows). (X30000). B. 2B: immuno-EM of rat uterus at the end of the 2nd week of gestation, labelled Ig G. showing black dots abundant inside lumen of the stromal blood capillaries, (L.B.C) (thin red arrows), and in the endothelial cells (E) (thin black arrow). (X30000).
Third Week Gestation: (Group 3)
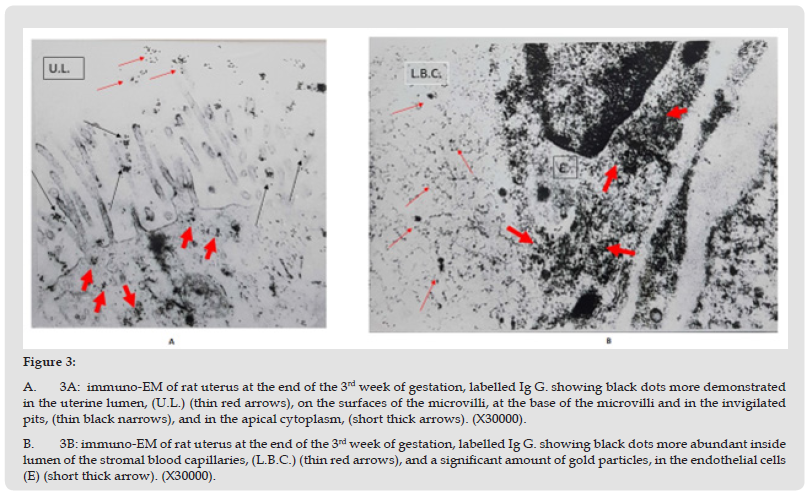
The amount of immunoglobulin G. labelled with gold particles was the highest at this week of gestation, large number of black dots of gold particles were seen in the uterine lumen, also they were localized on the microvilli of the luminal epithelial cells and on the apical invigilated pits at the base of the microvilli. Large number of gold particles were observed in the apical cytoplasm and along the lateral plasma membrane, (Figure 3-A). Significant amount of gold particles ware identified in the sub-epithelial stroma; in the stromal blood capillaries the gold particles were demonstrated in the endothelial cells as well as their lumen (Figure 3-B).
Figure 3 A. 3A: immuno-EM of rat uterus at the end of the 3rd week of gestation, labelled Ig G. showing black dots more demonstrated in the uterine lumen, (U.L.) (thin red arrows), on the surfaces of the microvilli, at the base of the microvilli and in the invigilated pits, (thin black narrows), and in the apical cytoplasm, (short thick arrows). (X30000). B. 3B: immuno-EM of rat uterus at the end of the 3rd week of gestation, labelled Ig G. showing black dots more abundant inside lumen of the stromal blood capillaries, (L.B.C.) (thin red arrows), and a significant amount of gold particles, in the endothelial cells (E) (short thick arrow). (X30000).
Our results confirm a close relationship between placental transfer of Ig G. and gestational age, it is found to be increase as the gestational age progressed this was in agreed by Pitcher-Wilmott, et al. [12], and Taslim, et al. [13], who previously reported that fetal Ig G. concentrations approximate maternal concentrations at 38 weeks and continued to increase to concentrations higher than maternal concentrations at delivery. The full-term infant therefore has an adequate level of Ig G. providing it with passive immunity to a wide range of potential pathogens. Malek, et al. [14] found significant correlation between Ig G. concentration in the fetal circulation and the gestational age with a substantial rise in the placental Ig G. transport capacity. Bernard, et al. [15], reported that in the days follow implantation, the embryo was bathed in immunoglobulin which was detectible in the trophoblast and in the extraembryonic endoderm, toward the end of pregnancy, Ig G. was transferred in significant amounts to the fetal circulation. For gestational age; Einborn, et al. [16], demonstrated that, neonates had lower (Ig G.) level than full-term infants. In the present study gold particles labelling (Ig G.) were detected in the uterine lumen in all gestational periods (groups 1,2, and ,3) reaching the maximum level at the end of gestation 3rd week, (group 3), this was in agree with results proved by, Wira, et al. [17], who detected immunoglobulin in the uterine lumen of rats, also, Rachman, et al. [18], demonstrated (Ig A.), and, (Ig G.) in the lumen of mouth uterus during the 1st half of pregnancy in close association of the embryo.
In the present study, gold particles detected in the luminal epithelial cells were seen to be localized at the surface membrane, at the apical invigilated pits, and in the cytoplasm, at the end of the 2nd week, gold particles were detected in the multivesicular bodies, and in apical vacuoles, they were more abundant on the end of the 3rd week, these results were in agree with the results reported in rabbits, [19], and, rats, [17].In the present study there was a definite labelling of Ig G. in the sub epithelial stromal cells of the blood capillaries and their lumen. Gold particles were more abundant at the end of the 3rd week, which was in agree with Bernard, et al. [15], who reported that as the mice embryo reaches the uterine lumen, the Ig G. was infiltrated into the stroma as a result of increase permeability of the uterine capillaries at time of implantation. Pippard, [20], reported that the stromal Ig G. probably was originated directly in the blood vessels and was accumulated in the tissues due to increase permeability of the blood capillaries at time of implantation. Neonatal immunization is largely unsuccessful due to immaturity of the infant’s immune system, therefore appropriate maternal immunization and passive transferred antibodies to the fetus is more efficient. Stanley, [21] reported that the concept of maternal immunization to protect the mother against vaccine preventable diseases and the neonate against illness for the 1st 3-6 months of life is a simple straight forwards and also more safe. Healy, et al. [22], reported that the maternal immunization with the passage of protective antibodies or immunoglobulin Ig G. to the infant is a potential strategy to prevent infection to the infants who have not completed their immunization from specific infection or vaccine preventable illness.


