Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Krsto Grozdanovski1 and Katerina Spasovska2*
Received: November 21, 2022; Published: December 21, 2022
*Corresponding author: Katerina Spasovska, Professor of Infectious diseases, Head of Department for Intensive Care, University Hospital for Infectious diseases and febrile conditions, bul Majka Teresa br 17, 1000 Skopje, Republic of North Macedonia
DOI: 10.26717/BJSTR.2022.47.007554
In recent decades, Hantavirus associated outbreaks have led to public concern and have created a global public health burden as an emerging zoonoses. Hantaviruses belong to a family of Bunyaviridae that are hosted in small mammals as their natural reservoir. Humans get infected accidentally, usually by inhaling contaminated aerosols. Hantavirus spillover from natural hosts into human populations could be considered an ecological process, in which environmental forces, behavioral determinants of exposure, and dynamics at the human–animal interface affect human susceptibility and the epidemiology of the disease. Hantaviruses cause two major clinical syndromes: hemorrhagic fever with renal syndrome (HFRS) in Europe and Asia and Hantavirus cardiopulmonary syndrome (HCPS) in the Americas. Approximately 150,000 to 200,000 cases of HFRS are hospitalized each year worldwide, with most of the cases occurring in developing countries. The case fatality rate of HFRS varies from 1-12% depending of the viruses. HCPS is rarer than HRSV, with approximately 200 cases per year, but with much higher fatality rate up to 40%. The central phenomena behind the pathogenesis of both HFRS and HCPS are increased vascular permeability and acute thrombocytopenia. The pathogenesis is likely to be a complex multifactorial process that includes contributions from immune responses, platelet dysfunction and the deregulation of endothelial cell barrier functions. As there is no effective treatment or vaccine approved for use in the USA and Europe, public awareness and precautionary measures are the only ways to minimize the risk of Hantavirus disease.
Keywords: Hantavirus; Hemorrhagic Fever with Renal Syndrome; Hantavirus Pulmonary Syndrome; Rodent Contro; Vaccine
Abbreviations: ANDV: Andes Virus; DOBV: Dobrava Virus; ELISA: Enzyme -Linked Immuno Essay; HV: Hanta Viruses; HTNV: Hantaan Virus; HFRS: Hemorrhagic Fever with Renal Syndrome; HCPS: Hantavirus Cardiopulmonary Syndrome; IFA: Indirect Immunoassay; LANV: Laguna Negra Virus; NE: Nephropathia Epidemica; PRNT: Plaque Reduction Neutralization Test; SAAV: Saaremaa Virus; SEOV: Seoul Virus; SNV: Sin Nombre Virus
Hantavirus syndromes are rare, but potentially life-threatening viral diseases associated with significant morbidity and mortality worldwide [1,2]. Hantaviruses belong to a family of Bunyaviridae and are hosted in small mammals. Humans get infected by either inhaling virus-contaminated aerosols or through contact with infected rodent droppings. There are more than 28 recognized Hantaviruses causing two major clinical syndromes: hemorrhagic fever with renal syndrome (HFRS) in Europe and Asia and hantavirus cardiopulmonary syndrome (HCPS) in the Americas [3,4]. Today, Hantaviruses are recognized as globally emerging zoonoses due to their significance as human pathogens with ability to cause outbreaks [5]. In this review, we reported what is so far known about Hantavirus syndromes, their epidemiology, ecology, diagnosis and treatment options as well as exsisting prevention meassures. In lack of ethiological therapy, our aim was to stress the importance of invention and immplementation of effective preventive strategies since new and potentially deadly serotypes are routinely being reported worldwide.
History
Hantavirus infection was first described in Chinese writings more than 900 years ago, but it attracted the world attention during Korean conflict (1950-53) when more than 3000 Korean and American soldiers fell ill with an infectious disease characterized by renal failure, fever, generalized hemorrhage and shock, with high lethal ity over 10%. It was called Korean hemorrhagic fever which today is commonly referred as hemorrhagic fever with renal syndrome (HFRS). Its etiological agent was identified 20 years later, in 1976, when Lee at al. isolated the virus from the lungs of the striped field mouse (Apodemus agrarius) and was named Hantaan virus [6]. The milder form of HFRS, nephropathia epidemica was known since early 1930s, but its causative agent Puumala virus was described in 1980, after it was isolated from the bank voles (Myodes glareolus) in Finland [7]. Another important fact in the history of HV infection is the outbreak that occurred in 1993 in USA, with HCPV caused by Sin Nombre virus, which was the first Hantavirus caused outbreak described in the Americas [8].
Etiology
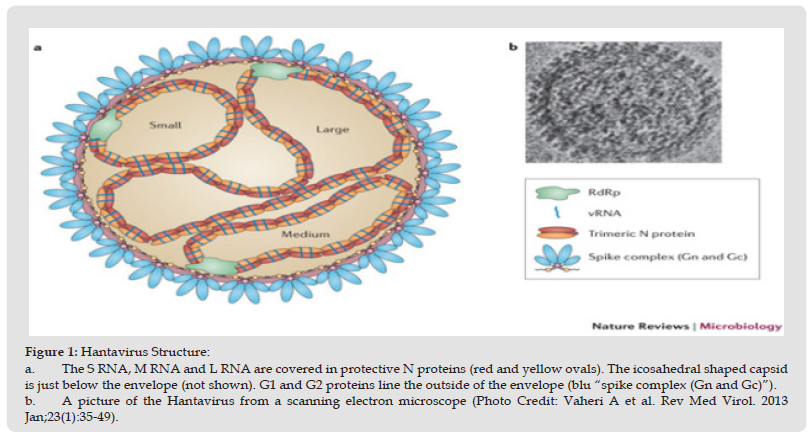
Hantaviruses are enveloped, spherical in shape RNA viruses that form a separate genus within Bunyaviridae family. The genome consist of three negative-sense, single-stranded RNA that share 3` terminal sequence of the genome segments. The three segments (small, medium and large) encode the nucleoprotein, envelope glycoproteins and the L protein or viral RNA-dependent polymerase (Figure 1) [9]. Hantavirus infects endothelial, epithelial dendritic and lymphocyte cells by attachment of viral glycoprotein with cell receptors (β1 and β3 integrin). It replicates in the cell cytoplasm, primarily bud with the Golgi complex. Most Hantaviruses have similar life-cycle however recent studies report that they evolved differently due to specific interactions with the host cell [10-12].
Figure 1 Hantavirus Structure: a. The S RNA, M RNA and L RNA are covered in protective N proteins (red and yellow ovals). The icosahedral shaped capsid is just below the envelope (not shown). G1 and G2 proteins line the outside of the envelope (blu “spike complex (Gn and Gc)”). b. A picture of the Hantavirus from a scanning electron microscope (Photo Credit: Vaheri A et al. Rev Med Virol. 2013 Jan;23(1):35-49).
Ecology
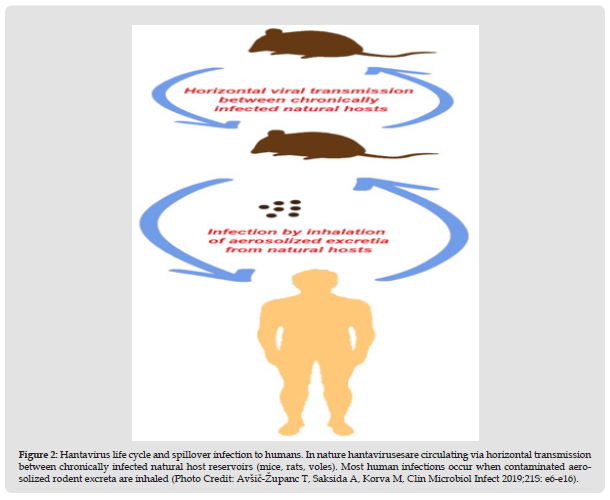
In the nature, Hantaviruses are circulating via horizontal transmission between chronically infected natural host reservoirs (mice, rats, voles). Usually the virus does not cause a disease in its natural host, but it is excreted in the animal urine, feces and saliva for months. Most human infections occur accidentally, when contaminated aerosolized rodent excreta are inhaled (Figure 2) [2,13]. Hantaviruses are usually closely related with single rodent and insectivore species, which is a result from a co-evolution between virus and the host during a million of years [14]. Infection of other animals is considered to be a spillover with minor or no risk for human infection. But spillover infection with a sympatric host seem to be a common incident that enables reassortment and origination of new Hantavirus species, that should be of importance for further following and public health awareness [15-17]. The ecology and geographical distribution of Hantaviruses are related to the distribution of their natural reservoir. According to their geographical distribution, there are two major groups, Old World Hantaviruses that circulate in Europe and Asia, and New World Hantaviruses, that dominate mainly in the Americas [2]. Old world hantaviruses are carried by four rodent genera: Myodes, Microtus, Apodemus and Ratus and by two insectivore families: Soridae and Talpidae [13]. In Europe, the most prevalent rodent reservoir for hantaviruses that inhabit the whole continent except Mediterranean region is the bank vole Myodes glareolus,and carry the Puumala Virus [18]. Hantavirus carried by Apodemus mouse that populate Europe and Asia causes a severe form of HFRS. In Asia Apodemus agrarius and Apodemus peninsulae are reservoirs for Hantaan and Hantaan-like viruses (Amur/Soochong virus) that populate Far East Russia, China and South Korea [19,20]. In Europe, in the Balkan region, severe form of HFRS with high mortality rate was reported in the early 1950s.
Figure 2 Hantavirus life cycle and spillover infection to humans. In nature hantavirusesare circulating via horizontal transmission between chronically infected natural host reservoirs (mice, rats, voles). Most human infections occur when contaminated aerosolized rodent excreta are inhaled (Photo Credit: Avšič-Županc T, Saksida A, Korva M, Clin Microbiol Infect 2019;21S: e6-e16).
Its causative agent, Dobrava virus was described in 1992 after isolation from its rodent host Apodemus flavicollis, in the region around Dobrava village in Slovenia [21]. Later, few Dobrava-like Hantaviruses were isolated from several Apodemus species that circulate all around Europe [22-25]. Seoul virus is another Hantavirus distributed all over the world due to by its natural host Rattus norvegicus [26]. In the Americas, first cases of hantavirus infection were described in 1993 after the outbreak of acute pulmonary distress syndrome in the Four Corner Areas of United States. The causative agent was named Sin Nombre virus which was isolated from its natural host, common deer mouse Peromyscus maniculatus [27]. Soon after, clinical cases of Hantavirus infections were confirmed also in Argentina, Bolivia, Brazil, Canada, Chile, Panama, Paraguay and Uruguay, isolated from different mice and rats that inhabit the continent [28,29]. The most prevalent Hantavirus in South America is Andes virus that causes HCPS and is the only Hantavirus that has ability for human to human transmission [30,31]. In Africa, first serological evidence for hantavirus infections among humans and rodents in was provided by Gonzales at al. in 1984 from survey in Benin, Burkina Faso, Gabon and Central African Republic [32]. The first African hantavirus, named Sangassou virus, was discovered in 2006 in Guinea, in its natural host, the wood mouse Hylomyscus simus [33]. Since then, hantavirus infections were reported surveys from several other countries such as Senegal, Nigeria, Djibuti and Egypt [34-37].
Epidemiology
In contrast to other genera from Bunyaviridae, hantaviruses are not transmitted to humans by arthropod but by direct contact with persistently infected rodents and their excreta. Humans are not natural hosts of hantaviruses and the infections usually occur accidentally by inhalation of virus containing aerosols from rodent’s excretions such as saliva, urine or feces [2,3,13]. Since 1996, human to human transmission is also considered possible after an inter- human transmission was demonstrated of Andes virus caused HCPS outbreak in Argentina [30,31]. The time and geographical distribution of hantavirus infections depend mainly on ecology and distribution of their rodent hosts. The risk for human infection is related to closeness of the contact and people who live or work with infected rodents are at highest risk. The infection is predominant in rural areas, although HFRS caused by Seoul Virus can also occur in urban areas. Occupation is the main risk factor and animal trappers, farmers, forestry workers, shepherds or military personnel are at highest risk. Epidemiological studies show link between viral exposure and activities such as heavily farm work, sleeping on the ground, military exercises and lower socioeconomic status. Both HFRS and HCPS occur more in male than in female, with M:F ratio 2:1 to 3:1, with predominance within 20-40 years age group [38- 40]. Today hantaviruses cause a significant number of human infections worldwide, making it and serious and emerge public health problem. It is estimated approximately 100 000 cases of HFRS annually in Eurasia and approximately 200 cases of HCPS in the Americas. Thus, many unrecognized, asymptomatic and non-specific mild infections result in an underestimated number of hantavirus infections worldwide [2,40,41].
Old World Hantaviruses
In Europe, most predominant hantavirus is Puumala Virus, responsible for approximately 9000 cases of HFRS annually. It occurs throughout the continent, predominantly in central and northern Europe, causing mild form of HFRS, Nephropathia epidemica (NE). Majority of cases are reported from Finland, Sweden and European Russia, although in the recent years it is also common in Norway, France, Belgium, Hungary, Austria and others [40-46]. Severe forms of HFRS in Europe are described mainly in the Balkan region and are caused by Dobrava virus, which natural host is Apodermus flavicolis. Several outbreaks of DOBV associated HFRS were reported in the region of former Yugoslavia since early 1950`s, with case fatality rate of 5-10% [41]. Both Puumala and Dobrava viruses are prevalent in the Balkans, similar to Central Europe, with higher incidence in the last years. A considerable antibody prevalence is reported in different Balkan countries (e.g 5% in Bosnia, 4% in Greece and 1,7% in Slovenia) suggesting that only some, probably most severe cases have been diagnosed [47-51]. Another Hantavirus carried by A.agrarius is SAAV that cause a mild form of HFRS with no fatality case. It is mainly reported in Russia, Germany and Slovakia [45,52,53].
In other European countries such as Italy, Latvia, Lithuania, Spain and United Kingdom, Hantavirus infections are demonstrated in sero-epidemiological surveys, but clinical cases have not been reported yet [54-58]. In Asia, clinical cases of Hantavirus infections are mainly reported in China, South Korea and Russia, although antibodies are found in rodents and in humans in Thailand, Indonesia and India. Most severe cases are caused by Hantaan virus and Amur/Sochong virus with mortality rate up to 15%. SEOV causes moderate form of the disease, with mortality rate of 1-2% [2,40,59]. China is considered to be major endemic region for Hantavirus in the world, with annual incidence of 40.000 – 60.000 cases that represent 70-90% of total HFRS cases in the world. More than 1.400.000 cases were reported over the period from 1950-2001, with 45.000 deaths, representing mortality rate of approximately 1%. These include cases of Apodemus–borne HV and Amur/Sochong virus as well as of rat-borne SEOV, but since comprehensive preventive strategies are implemented in China, the incidence rates of Hantaviral infections are decreasing in the recent years [60,61].
New World Hantaviruses
Approximately 200 cases of Hantavirus cardiopulmonary syndrome are reported annualy, both in North and South America, with mortality rate of around 40%. At least 15 Hantaviruses are identified as etiological agents of HCPS in the Americas; Sin Nombe virus is most predominant in USA and Andes virus in South America. ANDV is the only Hantavirus that has ability for human-to-human transmission and high mortality rate [62,63]. Although antibodies against Hantaviruses responsible for HFRS in Euroasia are detected in humans and rodents in the Americas, they may cause unrecognized, asymptomatic or subclinical forms of infection, since no clinical cases have yet been reported. First cases of Hantavirus infection in United Stated was described in May 1993, when previously healthy individuals from rural parts of so called “Four Corners” region of the southwestern United Stated died of acute respiratory distress syndrome. Their illness rapidly progressed from fever and myalgias to a respiratory failure with noncardiogenic pulmonary edema and hypotensive shock. Approximately 2 weeks after receiving laboratory specimens from the patients, public health investigators identified a newly recognized Hantavirus, Sin Nombre and its reservoir Peromyscus maniculatus [64,65]. Since the first outbreak in 1993 till today, Hantavirus infections are confirmed in 36 states of USA, mainly in the rural areas of western half of the country. As of January 2017, a total of 728 cases of Hantavirus disease have been reported in the USA, most of them in New Mexico and Colorado, with approximately 36% of deaths [66,67].
In South America, Hantavirus infection is becoming a huge public health problem since increasing number of cases reported each year. The first cluster of HCPS outside USA was described in Paraguay from July 1995 through January 1996, when 17 cases of HCPS caused by Laguna Negra Virus (LANV) were confirmed in people from western part of the country [68]. Today, cases of Hantavirus infections are identified all over the country, with average mortality rates of 15%. ANDV-like viruses are shown to be endemic in this region [69]. The first outbreak of HCPS in Argentina was reported in 1996 in the city of El Bolson, nestled in Andy mountings, when 18 cases were registered. During this outbreak, three doctors who treated ill patients also got sick, that suggested person to person transmission, which till than was not described for Hantaviruses [70]. Since then, several outbreaks were reported in Argentina, reaching mortality rate to 21,5% from 2009 to 2017. Pathogenic type of ANDV is most prevalent and endemic in this region [71].
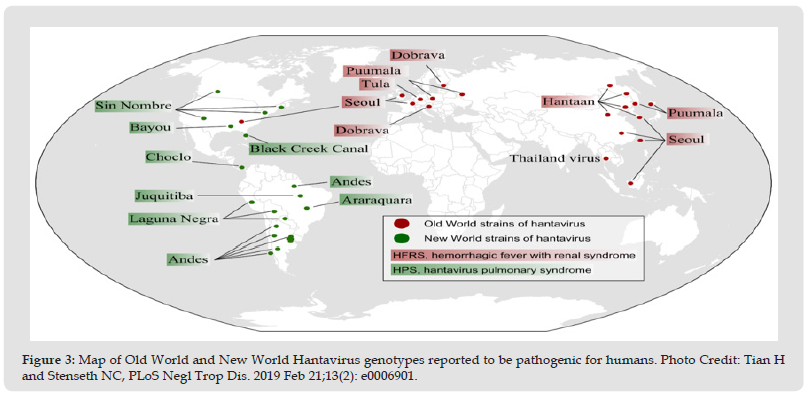
In Chile, in 1997, a cluster of 25 HCPS cases was reported. Important for this outbreak is that three children were infected and one of them died. Although it is difficult to isolate virus from the infected humans, the first human isolate of ANDV was from serum of an asymptomatic 10-year-old Chilean boy who died 6 days later [72]. A prospective study from Chile suggested that the risk for human- to-human transmission is higher among close household contacts of index patient with ANDV infection [73]. The first outbreak of HCPS in Central America occurred in 1999 to 2000, in Los Santos in Panama, with 12 cases and 25% mortality rate. A molecular research of viral genome identified new Hantavirus responsible for this outbreak, and was named Choclo virus. After the analysis of the rodents in the affected area, Oligoryzomys fulvescens showed to be a reservoir of this novel virus [74]. In the Caribbean region, a single case of HCPS was serologically confirmed in the eastern part of Venezuela, although infections are identified throughout the country [75]. First case of HCPS in Canada was reported in 1989, but Hantavirus infections are rare in this region, with less than 8 cases per year, and mortality rate up to 26% noted in North Alberta, Canada [76] (Figure 3).
Figure 3 Map of Old World and New World Hantavirus genotypes reported to be pathogenic for humans. Photo Credit: Tian H and Stenseth NC, PLoS Negl Trop Dis. 2019 Feb 21;13(2): e0006901.
Both syndromes, HFRS and HCPS are diagnosed based on clinical characteristics, epidemiological data and laboratory findings, but the diagnosis can be only confirmed by serological and molecular test [77-79]. There are varieties of clinical presentation of Hantavirus infections, from mild to severe forms, and early symptoms are non-specific. Most frequently patients present with high fever, headache, abdominal and back pains, conjunctival hyperemia, and gastrointestinal symptoms such as nausea, vomiting and diarrhea [80,81]. These clinical signs accompanied with laboratory findings of thrombocytopenia, leukocytosis, elevated levels of serum procalcitonin and C-reactive protein, increased serum creatinine, proteinuria and hematuria, should alert physician of possible Hantavirus infection. In addition, elevated alanine-aminotransferase and asparat- aminotransferase, as well as coagulopathy are also common laboratory findings [82-84]. Bilateral and diffuse lung interstitial rales in chest radiographs evolving to alveolar rales are found in patients with HCPS [85]. However, neither clinical or laboratory parameters are specific for Hantavirus disease and the diagnosis should be confirmed serologically by detection of specific IgM and IgG antibodies. Almost all patients have detectible high levels of virus specific antibodies at the onset of symptoms. Several tests are available for detection of IgM and IgG antibodies, but most commonly used are enzyme immunoassay (ELISA) based on recombinant nucleocapsid protein as well as indirect immunofluorescence assay (IFA), although the second one is with lower specificity. Immunoblot tests can be also used, but their results are in generally in agreement with those of ELISA or IFA tests for IgM antibodies [86-88]. Commercially available is also a rapid immunochromatographic test for detection of IgM antibodies that provides result in 5 minutes [89,90].
Although ELISA is optimal for serological confirmation of Hantavirus infection, there is a high level of cross-reactivity between different Hantaviruses, which indicates that serological tests cannot be used for virus serotyping. The plaque reduction neutralization test (PRNT) is considered to be a gold standard serological test that can be used for discrimination of various serotypes of Hantavirus. However, this test requires laboratories with biosafety level three that is a serious limitation for routine use [91]. Several molecular tests are available today for Hantavirus confirmation and genotypisation. Reverse transcriptional polymerase chain reaction test (RT-PCR) as well as one step assays based on real-time PCR proved to be with high level of specificity and sensitivity [92-94]. These tests detect viral RNA from blood, serum, urine, cerebrospinal fluid or saliva even before IgM antibodies, and are used in patients with suspected Hantavirus infection when rapid diagnosis is essential, especially in those with fast-evolving disease such as HCPS [95].
Currently there is no specific therapy available for Hantavirus infections; therefore the treatment is based on supportive measures. It is recommended that patients with HCPS and with severe HFRS to be admitted in the intensive care units for close monitoring and care. Maintaining the fluid and electrolyte balance is very important and amount of diuresis and kidney function must be carefully monitor according to patient`s fluid status to avoid over hydration. If shock develops, except fluid therapy, appropriate use of vasopressors might be indicated in both syndromes [96,97]. If severe thrombocytopenia and bleeding syndrome occur, blood transfusions should be used. Most patients with HFRS that have severe renal insufficiency need hemodialysis treatment [98]. Patients with HCPS usually need oxygen therapy and those who develop acute pulmonary distress are treated with mechanical ventilation. Extracorporeal membrane oxygenation (ECMO) is found to be useful rescue therapy for patients with severe HCPS [99].
Corticosteroids are not standard of care in treatment of Hantavirus infections. However, several studies report use of high dose methylprednisolone to treat severe HCPS, but there was no significant clinical benefit and further research for the efficiency of steroids in treatment of Hantavirus infections is needed [100,101]. At present no specific FDA approved drug is available for treatment of Hantavirus infections. Several candidate drugs have been evaluated in Hantavirus animal models [102]. Ribavirin (nucleoside analog) has shown antiviral activity both in vitro and in vivo, by several mechanisms that inhibit viral replication. Its anti-hantaviral effect was proven in treatment in suckling mice infected with HTNV. Clinical trials in China, in patients with HFRS, reported that Ribavirin significantly lowers mortality in patients if given during the first 5 days after onset of symptoms [103,104]. Rusnak at al, confirm that intravenous administrated Ribavirin in patients with HFRS reduces occurrence of oliguria and severity of renal insufficiency [105]. However, in so far conducted clinical trials, Ribavirin didn`t show significant efficiency in treatment and outcome of patients with HCPS [106]. A combination therapy of ribavirin and amixine (an interferon inducer) was tested in HFRS suckling albino mice proving that Hantaviruses are likely to be destroyed, that provides hope for development of new therapeutic strategies [107].
Lactoferrin (an iron-bindin glycoprotein) was reported to have broad spectrum of antiviral activity including Hantaviruses. In vivo, in SEOV infected suckling mice model, lactoferrin administrated at 2 doses of 160mg/kg and 40mg/kg, prior to virus inoculation, showed survival rate of 85% and 94% respectively. Lactoferrin also increases immune response by enhancing the cytotoxic activity of monoclonal cells and natural killer cells [108]. Favipiravir (a pyrazine derivative) is antiviral drug that inhibits RNA-dependent RNA polymerase mainly in influenza viruses but was tested and showed in vitro and in vivo efficiency against several bunyaviruses and arenaviruses, including Hantaviruses. It reduces viral RNA in blood and in the lungs in tested hamster models. But as in other studies, delayed administration of antivirals, after onset of viremia has showed no efficiency [109]. ETAR (1-β-D-ribofuranosyl-3-ethynyl-[1,2,4] triazole) is a nucleoside analog that has in vitro antiviral activity against HTNV and ANDV causing HFRS and HCPS. In vivo activity was confirmed after 10- and 15-days treatment of HTNV infected mice with ETAR 12,5mg/kg and 25mg/kg respectively, resulting in statistically significant increased survival rate [110]. Vandetanib is tyrosine- kinase inhibitor, a small molecule drug that interacts with receptors of virus infected endothelial cells and reduces membrane permeability. It is tested in ANDV hamster model and showed delayed lethality and increased survival of 23% [111].
Immunotherapy
Several studies on Hantavirus infected animal models shows that passive administration of monoclonal neutralizing antibodies as well as polyclonal antibodies to HNTV can provide sufficient protection. In addition, administration of two recombinant monoclonal antibodies derived from ANDV infected convalescents protects ANDV infected hamsters from lethal disease [112,113]. Plasma generated from HCPS patients infected with ANDV and SNV, protect hamsters and deer mice from developing the disease [114]. However, there are no clinical trials yet, on use of immunotherapy for HFRS and HCPS in humans. Currently a development of polyclonal antibody product using Hantavirus DNA vaccines is in progress and can move towards Phase 1 clinical trial [115-117].
The most preventive measures for Hantavirus infections are linked with rodent control and reduced human exposure to rodents and rodent`s excretes [118-121]. CDC recommends rodent proofing of homes, reducing rodent shelters and food sources in and around human homes, eliminating rodents from homes and preventing them from entering the homes, avoiding contact with contaminated areas using standard precaution measures while rodent contaminated areas are cleaned, personal protection measures for persons who have occupational exposure and precautions for campers and hikers [67]. In addition to standard personal and rodent control measures, an effective vaccine can contribute to reduce the risk for Hantavirus infection especially for most at risk populations. Four kinds of vaccines from inactivated HTV and SEO viruses are used in China and showed to be well tolerated and effective [122]. In Republic of Korea, an inactivated HNTV vaccine (Hantavax) is used for years [123]. However, there is no FDA approved vaccine yet. Several clinical trials on recombinant and molecular vaccines against Hantaviruses are ongoing. The focus is on development of DNA vaccine since it offers an easy way to construct a multivalent vaccine and that it induces long-lasting humoral and cellular immunity [124]. Until vaccine development, prevention of Hantavirus infections should be based on rodent control and avoidance of human exposure to rodents and their excretions [125].
Hantavirus syndromes are potentially life-threatening viral diseases associated with significant morbidity and mortality worldwide. Today, they are recognized as globally emerging zoonoses due to their significance as human pathogens since newer serotypes are routinely being reported with ability to cause outbreaks. Hantaviruses cause two major clinical syndromes: hemorrhagic fever with renal syndrome and Hantavirus cardiopulmonary syndrome. The pathogenesis of both syndromes is likely to be a complex multifactorial process that includes contributions from immune responses, platelet dysfunction and the deregulation of endothelial cell barrier functions. As there is no effective treatment or vaccine approved for use in the USA and Europe, public awareness and precautionary measures are the only ways to minimize the risk of Hantavirus disease.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Materials
As this article is a literature review, data sharing is not applicable as no datasets were generated or analysed during the current study.
Competing Interests
The authors declare no competing interests.
Funding
Not applicable.
Contributions
KG and KS establish review idea, information collection and writing. All authors read and approved the final manuscript.
Acknowledgement
Not applicable.


