Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Payam Saadat1, Masoomeh Noorbakhsh2, Nahid Beladi Moghadam3*, Dmitry Babarykin4, Galina Smirnova4,5, Soheil Najafi2, Mostafa Hosseini6 and Abbas Mirshafiey2,7*
Received: December 12, 2022; Published: December 21, 2022
*Corresponding author: Nahid Beladi Moghadam, Department of Neurology, Imam Hossein Hospital, Shahid Beheshti University of Medical Science, Abbas Mirshafiey, Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran, Livonian Biotech Millennium Ltd, Riga, LV-1013, Latvia
DOI: 10.26717/BJSTR.2022.47.007552
Background: The α-L-guluronic acid (G2013) as a new anti-inflammatory drug with immunomodulatory property, patented (PCT/EP2017/067920), showed positive effects in experimental models of multiple sclerosis (MS). In this clinical trial, our aim was to evaluate 6 months efficacy and safety outcomes in MS treated patients with guluronic acid compared to the conventional drug.
Method: In a 6-month, randomized controlled, phase II trial, we enrolled patients who had secondary progressive multiple sclerosis (SPMS), were 24 to 59 years of age, with a score of 0 to 8.5 on EDSS (higher degrees indicate more disability), and who had two or more relapses in the previous year. Patients received orally two doses of 500 mg daily of α-L-guluronic acid. Endpoints has been measured with magnetic resonance imaging (MRI) and Expanded Disability Status Scale (EDSS) and compared to the conventional drug (disease-modifying drug), in a phase 2 trial of multiple sclerosis at the beginning and end of the study.
Results: A total of 25 (92.5%) of the G2013 treated patients completed the study. The annualized relapse rate was 1.25 in α-L-guluronic acid group, and 1.58 in the conventional drug group at the beginning of the study (P<0.158). G2013 decreased the disability progression over the 6-month period. G2013 had a better performance compared to the conventional drug regarding to MRI-related measurements. Also, this medicine had no particular side effects.
Conclusions: As compared with the conventional drug, guluronic acid (G2013) improved the risk of disability progression (based on MRI and EDSS assessment). (Registered clinical trials number, IRCT2017042313739N8).
Keywords: Guluronic Acid; G2013; Multiple Sclerosis; SPMS; Clinical Trial
Abbreviations: MRI: Magnetic Resonance Imaging; EDSS: Expanded Disability Status Scale; DMT: Disease Modifying Treatment; CVID: Common Variable Immunodeficiency; MPO: Myeloperoxidase; RBC: Red Blood Cell Count; RA: rheumatoid arthritis; AS: ankylosing spondylitis; WBC: white blood cell; PLT: platelet count; Hb: hemoglobin concentration; MCHC: mean corpuscular hemoglobin concentration; EAE: experimental autoimmune encephalomyelitis; MCV: mean corpuscular volume; MCHC: mean corpuscular hemoglobin concentration; ALP: alkaline phosphatase; BUN: blood urea nitrogen; PGA: Physician’s Global Assessment; GPX1: glutathione peroxidase; G2013: guluronic acid
Multiple sclerosis is a chronic inflammatory disease of the central nervous system which results in the formation of focal convergent lesions of primary demyelination in the white and gray matter of the brain and to spread damage and neurodegeneration [1]. Generally, the disease starts in patients in the third decade of life with a relapsing and remitting clinical course. On average after 10- 15 years, in most of the patients, the illness alters to a course of slow progression (secondary progressive MS). There is a prominent difference between the relapsing and progressive stages of MS and this is also indicated by the various responses to currently available immunosuppressive or immunomodulatory treatments [2,3]. Nevertheless, there is an overlap in pathological characteristics, pathogenic mechanisms, and therapeutic responses between the relapsing and progressive MS [4-6]. Although several disease-modifying treatment (DMT) options exist for relapsing forms of this disease, we have limitations in DMT options for progressive MS (PPMS and SPMS) [7]. Alginates are natural copolymers composed of β-D-mannuronate and α-L-guluronate linked by 1→4 glycosidic linkages [8]. G2013 is an epimeric form of M2000 (β-D mannuronic acid) [9]. Guluronic acid (G2013), as an agent with low molecular weight has less toxicity on gastrointestinal tract and kidney function than the other anti- inflammatory medications, known as a novel drug, which could be categorized as a non-steroidal anti-inflammatory drug (NSAID), with immunomodulatory function [10,11].
The guluronic acid (C6H10O7) was obtained from alginic acid sodium salt and its IUPAC name is (2R/3S/4S/5S)-2/3/4/5-tetrahydroxy- 6-oxohex- anoic acid [12]. The guluronic acid showed its anti-inflammatory impacts by inducing SHIP1 and SOCS2 molecules, decreasing TLR4, MyD88, NF-kB and COX1/COX2 inflammatory enzymes at the level of gene expression and reducing the level of IL-1B as a pro-inflammatory cytokine [10,13]. In a clinical trial, Azarian, et al. [14]. approved that guluronic acid could be effective, safe and well tolerated in patients with rheumatoid arthritis (RA) and could be a proper choice in order to control the RA disease. This study showed that the oral use of G2013 in the dose of two capsules of 500mg per day in combination to conventional treatment in the majority of patients (72.7%) was led to a statistically significant increase in the ACR20 response rate after 12 weeks of G2013 treatment [15]. Another trial showed that the oral administration of G2013 was capable to modify the severity of articular and inflammatory symptoms of ankylosing spondylitis (AS) by decreasing the gene expression levels of the RORγt, IL-17, AHR, and IL-22 and increasing the gene expression levels of the GATA3, IL-4, and FOXP3 [16]. Afraei, et al. [14] demonstrated that G2013 moderate the clinical symptoms in experimental autoimmune encephalomyelitis (EAE), through suppressing NO production which resulted in a positive response than the control group, and this improvement has complied with clinical and histopathological demonstrations [17].
Their study has demonstrated its helpful effects with wonderful tolerability, efficacy and safety in EAE [14]. Furthermore, G2013 is able to decrease the number of inflammatory cells and plaques as well as the serum level of NO in G2013 dosed mice [18]. In this phase 2, conventional drug-controlled trial, we investigated the effects of daily G2013 treatment for six months using MRI measures for assessment of inflammation and disability progression in patients with secondary progressive multiple sclerosis.
Patients and Study Design
As previously described, this trial was a randomized, conventional drug-controlled, and phase 2 study of participants with SPMS diagnosed on the basis of the 2010 McDonald criteria. The patients who entered in this trial were aged 24 to 59 years and were injecting different forms of disease-modifying drug, also, patients who had at least one relapse in the previous 6 months or have active lesions in their MRI imaging and also had at least one T2-weighted brain lesion. Some exclusion criteria were a history of fever and infectious diseases, positive pregnancy test or lactation, collagen vascular diseases, other autoimmune diseases, malignancies, corticosteroid treatment within 30 days before randomization, other diseases (such as hepatic, renal, hematological, gastrointestinal, endocrine, cardiovascular, pulmonary, neurological or cerebral diseases). Patients signed the consent form before their enrollment in this clinical trial. The study (Clinical Trial identifier, IRCT2017042313739N8) was designed for the comparison of the efficacy and safety of guluronic acid (G2013) with conventional drugs over 6 months in the patients with SPMS. The protocols were reviewed and approved by regulatory authorities and the ethics committee of Tehran University of Medical Sciences (Tehran, Iran) and the study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. After confirmation that the patients fulfilled the defined criteria, they were randomly assigned at a 1:1 ratio.
Patients were randomly allocated to receive oral G2013 capsules in a dose of 500 mg, twice daily for 6 months. Two neurology centers in two different provinces (Tehran and Mazandaran) in Iran completed all the EDSS scores regarding SPMS patients. MRI scans were interpreted at the MRI assessment center by radiologists who were unaware of the study-group goal.
Safety Assessments
To assess the safety of the guluronic acid, the patients’ blood samples were obtained at each programmed visit. The hematological indices, red blood cell count (RBC), total white blood cell (WBC), platelet count (PLT), hemoglobin concentration (Hb), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and differential leukocyte count were evaluated. In addition, the blood smears, stained with Wright Giemsa stain, were prepared from each participant who had undergone the hematological tests. Clinical chemistry tests included glucose, phosphorus (P), calcium (Ca), sodium (Na), potassium (K), glucose, triglycerides, total cholesterol, total serum protein, albumin, serum alanine aminotransferase (ALT), serum aspartate aminotransferase, alkaline phosphatase (ALP), blood urea nitrogen (BUN), blood creatinine, uric acid, and total bilirubin were determined on baseline, 1st, 3th, and 6th months. In addition to clinical parameters evaluation, at each visit, participants were examined and questioned about all adverse events (AEs) based on the practical guide to self-report questionnaires (Patient History Form), including; the measurement of vital signs, physical examination. In this trial, the safety endpoints profile was on the basis of the incidence and type of AEs, serious AEs, infection and changes in clinical laboratory (hematological and biochemical) indices from baseline to month 6. All determinants were recorded at all visits.
Efficacy Endpoints
Clinical evaluations were performed after selecting and randomizing the eligible patients. The EDSS score was determined at baseline and 6 months. Standardized MRI scans were received at the screening visit and at 6 months. In this clinical trial, the primary endpoints were the determination of MRI and EDSS score. To assess the clinical evaluation, the primary endpoint was the percentage of patients free from Gd-enhanced lesions at baseline and month 6. The secondary endpoints were the percentage of patients free from relapses over six months and safety measures. Other endpoints included the number of Gd-enhanced lesions at six months, the number of new/enlarging T2 lesions over six months, the proportions of patients free from new/enlarging T2 lesions over six months, the proportions of patients free from new MRI activity (Gd-enhanced lesions and new/ enlarging T2 lesions). The key secondary endpoint was confirmed as a decrease of at least 1.0 point in the EDSS score. Other secondary endpoints included degree of patients free from gadolinium-enhancing lesions, number of gadolinium-enhancing lesions, number of new or enlarged lesions on T2-weighted MRI scans, between baseline and month 6, and evaluation of blood indices for the safety assessment and adverse events according to study protocol at baseline, month 1, month 3 and month 6 (Tables 1-3).
Note: Magnetic resonance imaging (MRI); Expanded Disability Status Scale (EDSS), All values are expressed as means ±SD.
Note: Magnetic resonance imaging (MRI); Expanded Disability Status Scale (EDSS), All values are expressed as means ±SD. p value ≤ 0.05 is considered as statistically significant.
Note: Number of patients (%).
Statistical Analysis
The data analysis includes all SPMS patients enrolled in two groups who took drug G2013 twice daily and conventional drug for 6 months. The descriptive statistics used here are included mean and standard deviation. Frequency and percentage were also used to classify the data. In the baseline time, all of clinical parameters were not statistically different between intervention and nonintervention groups. Kolmogorov– Smirnov test was used to determine the normality of quantitative data. For normally distributed data, parametric tests including T test and paired t test were used, respectively, for between group and also before and after intervention comparisons. For non-normally distributed data, pair-wise comparison between groups was performed using Mann–Whitney U test. Statistical analysis was conducted using SPSS version 24 and statistical significance was considered with p values ≤ 0.05.
Study Population
Of the 54 randomized SPMS eligible patients, 27 were assigned to guluronic acid group and 25 to conventional group. From May 2017 through November 2017, a total of 54 patients randomly assigned to 2 study groups (Figure 1) at 2 authorized neurology centers in 2 provinces in Iran. Baseline characteristics were similar across in two study groups (Table 1). In total, 25 G2013-treated patients (92.5%) completed the 6-month study. Two patients in the G2013-treated group withdrew from the study; one patient withdrew consent for unknown reasons and one patient withdrew due to the lost of follow-up. Three patients in the conventional-treated group withdrew the study; one patient withdrew due to the lost of follow-up, one patient withdrew due to lack of efficacy and one patients withdrew due to AE. The reasons for treatment discontinuation are summarized in (Figure 1).
Efficacy
a) MRI endpoints: The changes in the primary clinical efficacy end-points over the 6 months of treatment with G2013 and conventional drugs are shown in (Table 2). The mean number of patients free of gadolinium-enhancing lesions decreased in participants who received G2013 compared with the conventional drug group after 6 months (P<0.052), the mean number of patients free of Gd-enhancing lesions in G- 2013 treated group at baseline was 29.2 and at month 6 was 26.2. Also the number of T2 lesions was reduced in patients treated with G2013 than with conventional drug group, the mean of T2 lesions at baseline was 28.5± and at month 6 was 27. Changes from baseline in mean number of gadolinium-enhancing lesions were not statistically significant compared with those receiving conventional drug.
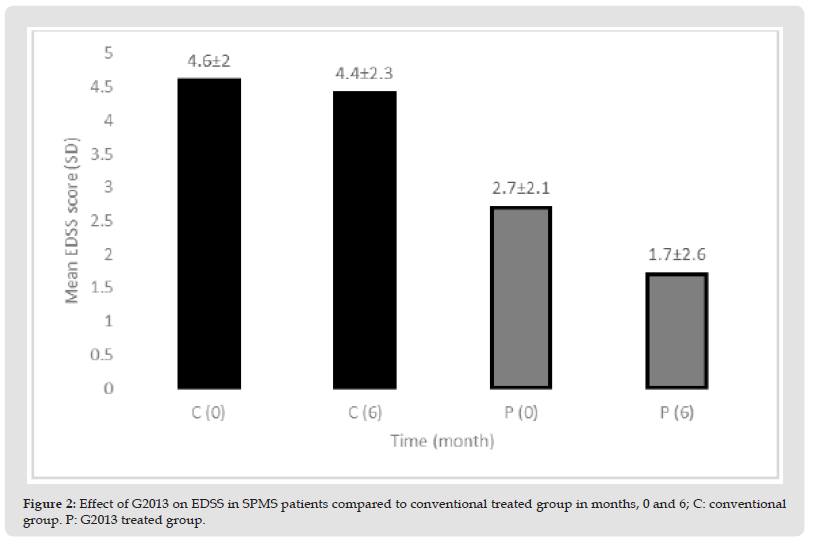
b) Clinical Endpoints: Changes in secondary clinical efficacy end-points after 6 months of treatment with G2013 and conventional drugs are shown in (Table 2). The administration of G2013 at 500mg twice daily (1000 mg/day) resulted in significantly reduced EDSS score compared to the conventional-treated patients after six months G2013 therapy. The mean of EDSS score at baseline in G2013-treated patients was 2.7 and at month 6 was 1.7. There was statistically significant improvement from baseline in EDSS score. The efficacy was sustained throughout the study and patients of the G2013-treated-group in comparison with patients of the conventional-treated group had achieved a significant response after six months of treatment. Disability progression of the disease in G2013 group was slower than patients who used the conventional drug. The α-L-guluronic acid reduced EDSS score in the sixth month vs. the conventional drug (P<0.009) (Table 2 & Figure 2).
Figure 2 Effect of G2013 on EDSS in SPMS patients compared to conventional treated group in months, 0 and 6; C: conventional group. P: G2013 treated group.
Safety
Safety data are summarized in (Table 3). Treatment with guluronic acid demonstrated an excellent safety and tolerability without notable adverse events (AEs) during the clinical trial. There were no patients in the G2013-treated patients that discontinued the study due to an AE, while one patient withdrew the study due to AEs in the conventional-treated patients. The biochemical and hematological data in G2013-treated patients did not show any treatment-related side effects. There were no statistically significant differences in the means of hematological and biochemical values at months 0, 1, 3 and 6 in G2013- treated patients (Tables 4,5). In addition, all of the mean values and individual amounts of the biochemical parameters in the G2013- treated patient’s sera approached to the normal range level during 6 months treatment. Also, it should be mentioned that the oral administration of guluronic acid had no-to-very low side effects, so that, there was no significant statistical difference between G2013-treated patients and conventional-treated group (Figure 3).
Figure 3 Effect of G2013 on Patients free of gadolinium -enhancing lesions in SPMS patients compared to conventional treated group in months, 0 and 6; C: conventional group. P: G2013 treated group.

This phase II trial has been done for the first time in order to compare the safety and efficacy of guluronic acid (G2013) as a novel NSAID with immunomodulatory property with conventional treatment in SPMS patient. The results of this trial provided that guluronic acid with 500 mg two times per day during six months of treatment of SPMS patients has significantly a superior efficacy when compared with conventional treatment.
Administration of NSAIDs with their side effects and ability to inhibit the progression of autoimmune diseases would be preferable. G2013 is an anti- inflammatory agent with therapeutic effects and excellent tolerability in animal models of multiple sclerosis. It is a safe drug with a low molecular weight and no toxicity on GI tract and kidney function [16,17]. In agreement with our findings provides evidence that G2013 as a derivative of alginic acid in addition to having therapeutic effects in MS patients; it could also decrease and lessen the GI and hematological complications. Therefore, it might be suggested that G2013 is a suitable option for the longterm management of MS. It has been shown in a study that the oral administration of G2013, as a new NSAID, was able to improve the inflammatory symptoms of ankylosing spondylitis patients through decreasing the inflammatory mediators [16]. Moreover, the low and high dose of G2013 could significantly reduce the gene expression of COX-1 and COX-2 enzymes in healthy volunteers [13].
Findings have demonstrated that G2013 as an immunomodulatory drug can decline inflammatory cytokine and be introduced as novel safe anti-inflammatory drugs in future [19,20]. The recent research showed that guluronic acid has the potential for managing autoimmunity in common variable immunodeficiency (CVID) patients [21,22]. Taeb, et al. [12] showed that G2013 can normalize the gene expression of myeloperoxidase (MPO) and might reduce the pathologic impacts of age-related inflammatory diseases by adjusting the expression of GPX1, SOD2, GST, CAT, and iNOS as the oxidative stress enzymes [12]. Another study by Mirshafiey, et al. [23] demonstrated that the expression of antioxidant enzymes that play a vital role against aging and age-related diseases like glutathione peroxidase (GPX1), super- oxide dismutase 2 (SOD2), catalase (CAT), glutathione-S- transferase (GST) was increased in G2013-treated rats in comparison with the control group [23]. Moreover, in a phase II clinical trial in patients with rheumatoid arthritis, it has been demonstrated that the oral use of G2013 in the dose of two capsules of 500 mg daily in combination to conventional treatment in the most patients (72.7%) resulted in a statistically significant increase in the ACR20 response rate after 12 weeks of G2013 therapy.
Additionally, the G2013-treated patients indicated a significant reduction in DAS28, tender joint counts, swollen joint counts, the Patient’s Global Assessment of Disease Activity (PtGA), Physician’s Global Assessment of Disease Activity (PGA) and lessening the inflammatory markers like CRP and ESR after 12 weeks of G2013 therapy [14]. In the present trial, due to our previous preclinical assessment, the approved dosage of G2013 was considered with the amount of 25mg/kg/d [22]. Although, in this trial we administered the minimum dose of G2013 (18 mg/kg/day), it is likely that a more efficient and a quicker onset of efficacy could be acquired with a higher or different dose of G2013. Our results showed that the mean EDSS score at baseline in G2013- treated patients was 2.7 and at month 6 was 1.7. There was statistically significant improvement from baseline in EDSS score. Also, the mean number of patients free of gadolinium-enhancing lesions decreased in participants who received G2013 comparing with the conventional drug group after 6 months (P<0.052). In conclusion, the oral α-L-guluronic acid than conventional drug had better efficacy during this 6-month trial in SPMS patients. MRI evidence in SPMS patients free of gadolinium- enhancing lesions and the number of T2 lesions were reduced with the use of G2013. Also, EDSS score in G2013-treated group comparing with conventional drug group was significantly reduced.
Additionally, G2013 therapy supported great tolerability and safety profile during the study and the incidences of AEs that happened during the clinical trial were in an acceptable profile in the G2013-treated patients. This clinical trial demonstrated that G2013 is beneficial, safe and generally well tolerated in patients with MS. The reported side effects of this drug are negligible. The long-term follow-up and increasing the number of patients is required for a better evaluation of the benefits and a precise assessment of its potent efficacy in multiple sclerosis disease.
The authors report that they have no conflicts of interest.


