Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
M Yasser Alsedfy*
Received: December 12, 2022; Published: December 20, 2022
*Corresponding author: M Yasser Alsedfy, Electronics and Nano Devices Lab, Faculty of Science, South Valley University, Qena, 83523, Egypt
DOI: 10.26717/BJSTR.2022.47.007548
Nanotechnology is considered as Modern scientific revolution which has a large scale of applications in many fields including the biomedical applications, nanoparticles like carbon nanotubes, and metals/metals oxides nanoparticles as titanium, zinc, iron, silver, gold are used in diagnosis and treatment, studies showed that a lot of these nanoparticles could affect our cells and organs badly due to its toxic effect, so it is important to raise our knowledge about this toxic effect, in this article we will discuss the reported toxic effect of iron/iron oxides nanoparticles as one of the most common used one type in many medical applications.
Abbreviations: ROS: Reactive Oxygen Species; CAP :Cold Atmospheric Plasma; GSCS: Glioblastoma Stem-Like Cells; GIT: Gastrointestinal Tract; LDH: Lactate Dehydrogenase; BALF: Bronchoalveolar Lavage Fluid; ALP: Alkaline Phosphatase; EPR: Electron Paramagnetic Resonance; SPIONs: Super paramagnetic Iron Oxide Nanoparticles; MRI: Magnetic Resonance Imaging; BBB: Blood–Brain Barrier; MIONs: Magnetic Iron Oxide Nanoparticles; ALT: Alanine Transaminase; AST: Aspartate Aminotransferase; ALP: Alkaline Phosphatase; ADH: Antidiuretic Hormone; PTH: Parathyroid Hormone; PAA: Poly Acrylic Acid; TEM: Transmission Electron Microscopy ; GFR: Glomerular Filtration Rate
Nano-toxicology is a branch of science which concern about the toxicity of nanomaterials and its negative effects on human health. Although some researchers are against utilizing Nano medicines some others are in favor believing that their side effects are acceptable. Iron oxides nanoparticles are wildly used in treatments and detection, it has been used in hyperthermia as cancer therapy, imaging, targeting drug delivery and medical imaging. There are many studies which focused on the toxic effect of (IONPs), concluding that the cytotoxicity of IONPs is linked to many factors like particles size, surface coating, surface chemistry and dosage [1]. Nowadays, there are several FDA-approved magnetite-based NPs including Umirem- VR, FeridexVR, GastroMARKTM, GastromarkVR, FerahemeVR, and Feridex I.V.R, as well as numbers of clinical trials on other IONPs forms [2]. Potential mechanisms of SPIONs toxicity can be summarized in producing reactive oxygen species (ROS) in vivo, which induces mitochondria-mediated apoptosis, and inflammation. In this article we will go through the expected scenario of IONPs interaction with our body beginning from oxidative stress exerted on our cells [3].
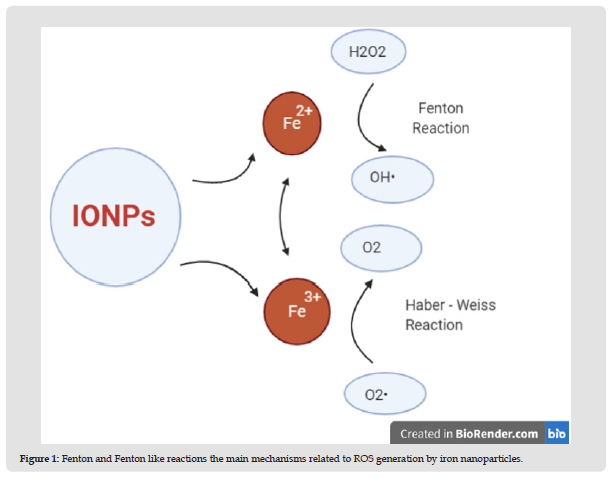
Oxidative stress in simple words can be defined as an imbalance between the production of free radicals and the ability of the body to overcome their harmful effects through neutralization by antioxidants, Oxidative stress is caused by exposure to reactive oxygen intermediates, such as superoxide anion (O-), hydrogen peroxide (H2O2), and hydroxyl radical (OH) [4], It is well documented that reactive oxygen species (ROS) production may be the main cause of Iron-based nanoparticles toxic effect on the cell, Continuous increase in the concentration of (ROS) Resulting genotoxicity or intracellular signaling which may lead to reduced cell viability and apoptosis. It is possible that Fenton and Fenton-like reactions (Figure 1) are the main mechanisms related to ROS generation by iron nanoparticles [5].
Figure 1 Fenton and Fenton like reactions the main mechanisms related to ROS generation by iron nanoparticles.
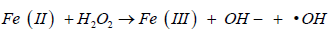 (classic homogeneous Fenton reaction)
(classic homogeneous Fenton reaction)
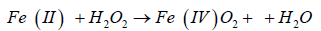
(non-radical process)
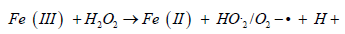 (homogeneous Fenton like reaction)
(homogeneous Fenton like reaction)
The Haber-Weiss reaction can also be a significant contributing factor:
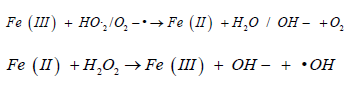
Although ROS regulate the normal activities of the cell, an abnormal increase of ROS levels may damage the cell, ROS also makes the outer mitochondrial membrane more permeable and can damage the lysosomal membrane Furthermore, ROS can affect DNA by several mechanisms include degradation of bases, breaking of DNA chain, mutations, changing DNA composition, deletions or translocations, and cross-linking with proteins. Which could lead to aging,cancer, and neurodegenerative diseases, ROS can initiate cellular injuries by modifying lipids, proteins, and DNA or lead to generating secondary ROS and finally cell damage [6]. The concentration and features of NPS, including size, shape, coating, and a functional group, and also cell type control NPs accumulation within the cell, for example, it was reported that dextran and PEG coatings reduce Iron oxide nanoparticle cytotoxicity with various size (5,30) nm while cell death induced by Bare nanoparticles in both sizes was 6fold higher [7]. Even the redox state of Iron-based nanoparticles can affect its cytotoxicity as shown in a study on Escherichia coli (Figure 2) [6].
Figure 2 Illustrating schematic for several toxic interactions of ROS caused by iron ions with intracellular components.
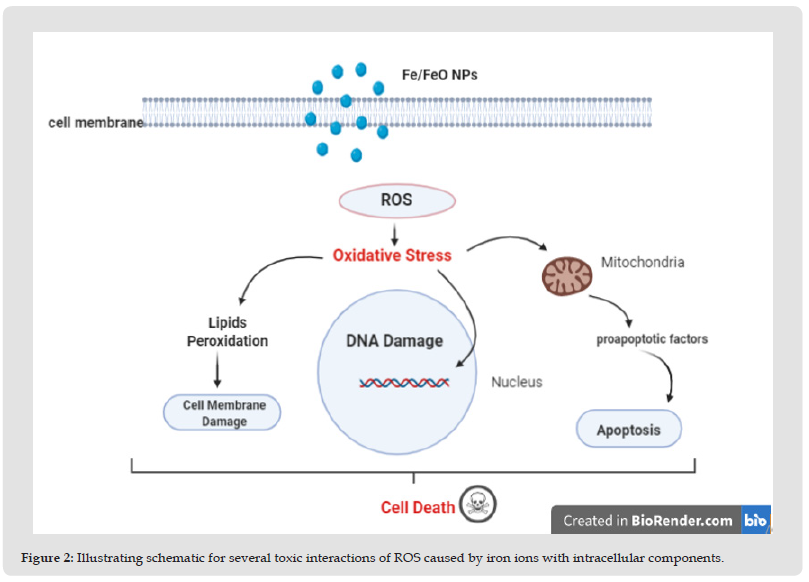
• Iron ions excess in the cytoplasm could lead to mitochondria damage and apoptosis induction by affecting on its membrane permeability accordingly Ca+2 ions and proapoptotic factors like cytochrome C and caspase 9 will be released [8], and induce cell programmed death [6,9].
• ROS which are produced by iron ions can interact with cell membrane causing lipid peroxidation and cell membrane damage.
• ROS could have a lethal damage to the DNA in several ways like breaking DNA chains and stimulating mutations occurrence resulting in DNA damage then apoptosis [10].
Apoptosis is the last stage of the prolonged cell damage caused by ROS, apoptosis takes place after cell failure to repair DNA damage, researchers were inspired by induced oxidative stress and apoptosis caused by NPs to study its effect on cancerous cells. there are a lot of signaling pathways those contribute to cancer cells like Bax/Bad, P53, BCL-2, and others [11]. A Combination of cold atmospheric plasma (CAP) and iron NPs in human breast adenocarcinoma cells (MCF-7) was reported to reduce the viability of cancer cells significantly and induced apoptosis through shifting the BAX/ BCL-2 ratio [12]. another study in 2015 reported that functionalized IONPs conjugated with cetuximab induced apoptosis in glioblastoma stem-like cells (GSCs) with a significant antitumor effect that was greater than cetuximab alone [13] a recent study reported that INPs can activate the activities of caspase-3, caspase-8, and caspase-9 which means that the functionalized iron nanoparticles induce apoptosis in MCF-7 breast cancer cell lines and could be an opportunity in the future to use the iron nanoparticles for stimulating apoptosis in cancer treatment [14]. however the cytotoxic effect of IONPs on the malignant cell is documented in various studies it still unclear if it is the same effect on normal cells or all types of the cancerous cells so that additional focusing studies are needed to give us better insights on antitumor effect applications of IONPs [11]. on the other hand, A lot of studies are concerned with the relation.
Between oxidative stress caused by Iron-based nanoparticles and diseases like neurodegenerative diseases especially. Such as Alzheimer’s disease and Parkinson’s disease that result from alterations in brain iron homeostasis [15-17]. In contrary Umarao et al. declared that iron oxide nanoparticle implantation and magnetic field exposure in an in vivo 6-OHDA rat model of Parkinson disease could have a neuroprotective effect. more studies should be conducted to understand more about the effect IONPs on various types of cells and organs because the lack of studies and the conflicting results are confusing so far [18].
Inhalation is the most relevant exposure route for occupational exposure to nanoparticles. Nanoparticles disposition within the lung is a complex function of the kinetics of absorption and non-absorptive clearance mechanism [19] Inhaled nanomaterials which are insoluble in mucus and lining fluid in the respiratory system, are not able to be rapidly absorbed and may undergo physical translocation. It depends on the lung region in which the nanoparticles have been deposited [20] Once the particles reach the bronchi, they will be transported up into the pharynx and swallowed, causing gastrointestinal tract (GIT) exposure Nonetheless, the uptake of NPs by the GIT has been shown to be very low Inhaled NPs may also migrate across the alveolar epithelium, through interstitium, access systemic circulation directly or via lymphatic vessels and accumulate in the liver and spleen [21] particle inhalation is an important exposure mechanism [22]. Despite the importance of nanoparticles inhalation toxicity assessment, we found a few of studies and lake of data that can demonstrate acute and chronic exposure effect of NPS on airways in animals. a continuous 4 h inhalation exposure of only the head and nose to iron oxide nanoparticles (Fe3O4 NPs, with average size 15–20 nm) was investigated to assess the acute effect. The rats exposed to a concentration of 640mg/m3 Fe3O4 NPs, the cell viability significantly decreased, the levels of lactate dehydrogenase (LDH), total protein, and alkaline phosphatase were elevated in the bronchoalveolar lavage fluid (BALF).
Also, total leukocyte count and the percentage of neutrophils in BALF increased within 24 h after exposure [23]. LDH is a cytoplasmic enzyme which is used to indicate cell injury [24]. The total protein content in BALF can also be used to indicate lung epithelial damage [25]. Alkaline phosphatase (ALP) in lung lavage fluids also is an indicator for tissue damage and type II cell proliferation [26], which means iron nanoparticles may induce cytotoxicity. Regarding to chronic exposure, we found only one study which assessed the long-term exposure toxicity via inhalation. it may be because of the difficulty to recreate such chronic exposures in vivo specially in rodent model according to a short lifespan of no more than two years, also the conclusions of such studies are not very accurate because of the limited period of investigation and limited nanoparticle sizes used in dosing usually with only one chemical composition and having dimensions within a narrow nanometric range. Fe2O3 NPs with the mean diameter of (14 ± 4 nm) Rats were exposed to these NPs for 4 h a day, 5 days a week during 3, 6, or 10 months at the mean concentration of (1.14 ± 0.01 mg/m3). The Fe2O3 content of the lungs and lung-associated lymph nodes was measured by the electron paramagnetic resonance (EPR) spectroscopy. a relatively low but significant pulmonary accumulation of Fe2O3 was found to be increased gradually with time [27] .
A very recent study which simulated a higher concentration of Iron oxide nanoparticles than the environmental level to investigate the toxicity of inhaled FeOxNPs of different forms (Fe3O4, α- Fe2O3 and γ-Fe2O3) through nose only exposure (50 μg/m3 and 500 μg/m3, 3 h/d × 3 d), there were no clinical signs of toxicity and demonstrated a lack of pulmonary toxicity at all [24].
Superparamagnetic iron oxide nanoparticles (SPIONs) are involved in a lot of applications in biomedical fields, including drug delivery carriers, magnetic hyperthermia and gene transfection agents because of their superparamagnetic properties [28], also iron oxide NPs and their conjugates are widely used as contrast agents in magnetic resonance imaging (MRI) [29], IONPs have gained significant interest for diagnostic and therapeutic applications in the central nervous system in recent years [30] because of Their magnetic and paramagnetic advantages and potentiality to penetrate the blood–brain barrier (BBB) which made the IONPs a promising neuroimaging platform for evaluating CNS tumor lesions, grading, differential diagnose, image-guided delivery in brain tumor treatment, detecting disease progression, monitoring therapies and design of specific treatment strategies for CNS disorders , and the list continues [31], We have found that there is lack of studies and there is controversy and disagreement over the safety profile of IONPs for example a study on adult male mice which were intraperitoneally administered with Fe2O3-NPs (25 and 50 mg/kg body weight) once a week for 4 weeks. Fe2O3-NP treated Rat’s Locomotor behavior and spatial memory change were observed. Damaged blood-brain barrier permeability Elevated nitric oxide, acetylcholinesterase, lactate dehydrogenase leakage, and demyelination were observed in the Fe2O3-NP-treated brain tissues. Imbalanced levels of ROS generation and antioxidant defense mechanism (superoxide dismutase and catalase) cause damages to lipids, proteins, and DNA.
PARP which is an enzyme that helps in repairing damaged DNA [32] and cleaved caspase 3 expression levels which is related to apoptosis [33] were found to be increased in the Fe2O3-NP-exposed brain regions which confirms DNA damage and apoptosis. This means impairments were caused by nanoparticle accumulation, oxidative stress, and apoptosis in the mouse brain. [31] , our brain is vulnerable to nanoparticles, a study on fine Fe2O3 particles (280±80 nm) suggested that it could transport into the CNS via the olfactory pathway by sensory nerve endings of the olfactory nerve and trigeminus [34], The striatum and hippocampus are important structures in the brain which are associated with the development of Parkinson’s and Alzheimer’s diseases another study reported that iron oxide nanoparticles (Fe3O4-NPs, 30 nm) were intranasally instilled for seven days have the ability to translocate directly from the olfactory nerve to the brain .the study focused on the effects of Fe3O4-NPs on the striatum and hippocampus, including oxidative injury and the accumulation and retention of Fe3O4-NPs. regional distribution of Fe3O4-NPs was observed in rat brains after treatment. The particles were found to be deposited at high concentrations in the rat striatum and hippocampus. Over half of the Fe3O4- NPs were retained in the striatum for a minimum of two weeks and may have induced oxidative damage to the region. Also in vitro studies showed that Fe3O4-NPs may decrease neuron viability, trigger oxidative stress, and activate JNK- and p53-mediated pathways to regulate the cell cycle and apoptosis.
Which can be considered as an eye-catching study to intensify the study of IONPs environmental exposure to know if it can develop neurodegenerative diseases [35], a low-dose (130 μg) repeatedly intranasal instilled nano- and submicron-sized Fe2O3 particles (21 nm and 280 nm) to mice showed to induce significant oxidative stress by both of sizes of Fe2O3 particles. From the oxidative stress biochemical measurements and the ultrastructural changes of hippocampus and olfactory bulb nerve cells, the results indicated that the 21 nm-Fe2O3 particles may induce more severe oxidative stress and cell injury more than 280 nm-Fe2O3 which means it’s a size-dependent toxic effect. the study revealed the oxidative stress effect of IONPs on CNS and it’s neurotoxic impact resulting from long-term inhalation exposure [36]. another in vivo study investigated The toxicity of magnetic iron oxide nanoparticles (MIONs) by measuring iron content in different brain areas of Wistar rats, spherical shape MIONs which were (15.63 ± 2.38 nm) in size used as a single i.e. injection in a dose of 10 mg/ kg delivered to rats, this study demonstrated that the toxic effect of (MIONs) varies from one area to another of rat brains. the hippocampus and striatum were the most affected areas and showed severe damage due to MIONs exposure, on the contrary thalamus and cerebellum were The least affected areas and based on The histopathological examination of the brain, moderate neuronal degeneration in hippocampus and striatum, mild neuronal degeneration in the cortex and slight degeneration in hypothalamus and pons-medulla areas were detected [37].
On the other hand, some studies declared the safe use of based IONPs clinical magnetic resonance contrast agents like ferumoxtran- 10, ferumoxides, and ferumoxytol in the brain and approved its ability to provide imaging parameters and time course data in brain tumors and neurological lesions studies, Iron oxide clinical agents were administered by intracerebral inoculation or intraarterially after osmotic blood-brain barrier opening in normal rats and intravenously in nude rats with intracerebral tumor xenografts. Rat brains were imaged by MRI at multiple time points and then were assessed for iron histochemistry and pathological features. There were No pathological brain cell or myelin changes were detected after delivery of the clinical iron oxide agents to normal brains [38] . nevertheless some studies have called for more caution with using IONPs as contrast agent, specially in particular diseases conditions of neurodegenerative diseases, like multiple sclerosis and Alzheimer’s and Parkinson’s disease which were found to be linked to iron deposition in activated microglia leading to homeostatic imbalance and even cell death [39,40]. So that more studies should be done to assess adverse events related to IONPs especially with neurodegenerative diseases patients.
The liver is the most important detoxification organ in our body, it is reached by the highest level of NPS concentration, over all the other tissues. The liver, compared to other reticuloendothelial organs, seems to be the major organ for the accumulation of insoluble particles such as metal NPS after accessing systemic circulation. Translocated NPs reach the liver, and they tend to accumulate in the Kupffer cells, which are specialized macrophages located in the liver, The Kupffer cells are considered to be the first line of the defense against foreign bodies [21], liver enzymes Alanine transaminase (ALT) and aspartate aminotransferase (AST) are two of the most reliable markers of hepatocellular injury or necrosis. Their levels in blood serum can be increased in a variety of hepatic disorders. ALT is thought to be more specific for hepatic injury than AST, because it is present mainly in the cytosol of the hepatic cells and, in low concentrations, while The AST is present in tissues of the liver, heart, skeletal muscle, kidneys, brain, pancreas, lungs and in white and red blood cells. Alkaline phosphatase (ALP), which is present in a number of tissues, including liver, bone, intestine and placenta, is measured to detect the liver disease or bone disorders also It is known that NPs are primarily readily taken up by hepatocytes and Kupffer cells (specialized macrophages located in the liver) following their exposure. So that kupffer cells number is usually determined in hepatic nanotoxicology studies [41]. IONPs toxicity was suggested to be a dose-dependent.
In a study which indicated that, even though 150 and 300 μg/gr doses showed adverse effects on liver, the administration of Fe3O4- NPs in doses of 25, 50 and 75 μg/gr can be regarded as safe [42], since Most metal oxide nanoparticles show toxic effects, but a very low or no toxic effects have been observed with biocompatible coatings [43], some studies investigated the toxic effect of coated IONPs on the liver, a study conducted on adult male albino rats to compare between Polyethylene glycol coated IONPs and Bare IONPs showed that PEG-coated IONPs were less hepatotoxic than the bare [44]. the use of IONPs as MRI contrast agent with liver patients should be careful and with certain standards and dosages, A single dose of SPION injection at 0.5 or 5 mg Fe/kg delivered to the cirrhosis mouse group induced a septic shock response at 24 h with elevated serum levels of liver and kidney function markers, and significantly altered gene expressions of toxicity pathways and, impacts extended over 14 days including high levels of serum cholesterols and persistent low serum iron level. In contrast, full recovery of liver functions was found in the normal group with the same dosages over time this study also indicated that with a low dose of SPIONs at 0.5 mg Fe/kg in mice, which is equivalent to 0.4 mg Fe/kg for human based on the body surface area [45], there was no significant difference among the serum markers with or without injection after 14 days and the short-term septic shock type of responses were minimal [46].
Dextran is a biocompatible material extensively used in biomedical applications for coating nanoparticles to prevent the agglomeration and toxicity of magnetic particles [47], a study conducted on rats to assess the toxicity of intravenously single dose of 10 mg/ kg body weight DIONPs (1 ml test substance preparation in saline/ kg body weight) on animal’s liver, Analysis of hepatic enzymes and bilirubin levels further confirmed that DIONPs are well-tolerated and do not affect the liver functions [48]. A pre-clinical study to determine polyacrylic acid-coated iron toxic effects in CD-1mice using a single acute (8, 20, or 50mg/kg) intravenous administration of PAA-coated IONs in magnetite form showed that PAA-coated IONs tend to accumulate mainly in the liver and spleen and despite an inflammatory process was triggered, data showed that PAA-coated IONs do not cause severe organ damage [49].
In mammals, tshe kidneys are the main excretory organ, which eliminates metabolic wastes from the body. Kidneys have several functions in maintaining life. Conserve Water and many electrolytes in balance. conserve nutrients such as glucose and amino acids, maintaining blood PH. Producing several hormones, including renin, erythropoietin, and prostaglandins, The kidneys also respond to several hormones, including antidiuretic hormone (ADH), parathyroid hormone (PTH), aldosterone, thyroid hormone, and others [50]. Despite this vital role of the kidney less attention has been drawn to NPS nephrotoxicity. A study on, green magnetic iron oxide nanoparticles were prepared by using aqueous Desmodium gangeticum root extract as a reducing agent and labeled as (FeNP DG). Nephrotoxicity was initially evaluated in treated Wistar rats those were given once Injected with (100 mg/kg b.wt; intraperitoneally for 2 weeks. by histopathological assessment of kidney weight, and blood biochemistry indicators. A comparison between the toxic effect of FeNP versus the green synthesized FeNP (FeNP DG) showed that, morphological changes in the tubular and cortical region, were obvious. FeNP treated rat kidneys showed a diffused pattern of morphological changes, whereas FeNP DG treated animals exhibited minimum localized changes. In fact, these changes were marginal in the cortex and medullary regions of nephrons. In support of this finding, the relative organ weight measurement shows a significant increase in kidney weight of FeNP treated group as compared to the control and FeNP DG group.
Blood chemistry revealed significantly increased blood urea nitrogen (BUN). However, serum creatinine level was significantly decreased in both FeNP and FeNP DG, compared to the normal control. creatinine and its low clearance value was observed in FeNP and FeNP DG when compared with normal, indicate altered renal function. However, an elevated urea clearance was found in FeNP and FeNP DG treated rats compared to the normal animal. Relatively high serum urea/creatinine ratio was observed in FeNPs treated rats, alkaline phosphatase, alanine transaminase, and aspartate transaminase, were also significantly increased in the FeNP treated group, as compared with the FeNP DG and the control [51]. Another study describes the distribution of polyacrylic acid (PAA) coated γ-Fe2O3 NPs intravenously injected .using Magnetic resonance imaging (MRI) and transmission electron microscopy (TEM) showed intracellular uptake in renal cells, particularly the cytoplasm of the proximal tubule, in podocytes and mesangial cells. The renal functional effects of NPs were evaluated by arterial acid–base status and measurements of glomerular filtration rate (GFR) after instrumentation with chronically indwelling catheters. Twenty-four and 96 h after NP injections, the GFR averaged 0.35±0.04 and 0.35±0.01 ml min−1 g−1( , respectively, values which were statistically comparable with controls (0.29±0.02 and 0.33±0.1 ml–1 min–125 g–1).
This means that the accumulation of superparamagnetic iron oxide nanoparticles does not affect kidney function in healthy mice which is hopefully means contrast agents for magnetic resonance imaging is safe [52], but also more data is needed regarding renal impairment patients.
The skin is the largest organ of the body which represents more than 10% of body mass and plays a defensive role as a barrier against the external environment, absorption through the skin is an important route of entry for nanoparticles both in occupational and consumer settings. Most of dermal nanotoxicology studies focus on TiO2 and ZnO nanoparticles as widely used in cosmetics, we found only one study demonstrated that thrombin-conjugated γ-Fe2O3nanoparticles accelerated the healing of incisional wounds significantly in rats better than free thrombin [53]. We notice that there are very few toxicity in vivo and in vitro IONPs dermal toxicity studies.
In this mini review article the toxic effects of IONPs on our body’s organs were discussed and investigated, it is clear that IONPs could be toxic on our body’s cells, we can summarize, most in vivo findings and clinical evidence suggest that SPIONs can be considered as biocompatible and nontoxic particles, although administration of high doses of iron may accompany with the chronic iron toxicity in human, also a biocompatible coating for IONPs could reduce their toxicity.


