Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Prasun Guha1,2* and Hafiz Ahmed1,3*
Received: October 25, 2022; Published: December 20, 2022
*Corresponding author: Prasun Guha, Department of Biochemistry and Molecular Biology, University of Maryland School of Medicine and Institute of Marine and Environmental Technology, 701 East Pratt Street, Baltimore, MD 21202, US and Nevada Institute of Personalized Medicine, University of Nevada, Las Vegas, NV 89154, USA
Hafiz Ahmed, Department of Biochemistry and Molecular Biology, University of Maryland School of Medicine and Institute of Marine and Environmental Technology, 701 East Pratt Street, Baltimore, MD 21202, USA and GlycoMantra, Inc., 1450 South Rolling Road, Baltimore, MD 21227, USA
DOI: 10.26717/BJSTR.2022.47.007545
Tunicamycin mediated induction of endoplasmic reticulum (ER) stress promotes cell death of cancer cells. However, the apoptotic doses of tunicamycin shows immense toxicity to normal cells and thus limits its use as a cancer chemotherapeutic. Here, we showed that a low dose of tunicamycin for 24 h induced autophagy in PC3 prostate cancer cells without inducing cell death. Interestingly, when autophagy was blocked by lysomotropic drug chloroquine, same amount of tunicamycin caused synergistic cell death of PC3 cells as well as MDA MB436 and MDA MB231 breast cancer cells as evidenced by cell viability and TUNEL assays. Consistent with the in vitro data above, a combination of chloroquine and tunicamycin had a synergistic inhibitory effect on tumor growth of MDA MB231 cells that xenografted in nude mice. Taken together, our results suggest that a combination treatment of autophagy blocking with chloroquine and ER stress induction with low dose of tunicamycin could be a promising strategy for cancer therapy.
Keywords: Autophagy; Tunicamycin; ER Stress; Chloroquine
Abbreviations: ER: Endoplasmic Reticulum; ROS: Reactive Oxygen Species; Tun: Tunicamycin; CQ: Chloroquine; PBS: Phosphate-Buffered Saline (10 mM phosphate, 140 mM NaCl, pH 7.5)
Within the endoplasmic reticulum (ER), nascent proteins are appropriately folded through the actions of a series of molecular chaperones, lectins, and glycosidases and travelled to the Golgi apparatus for further trafficking [1]. When this process is incomplete or if there is an imbalance between the cellular demand for protein synthesis and the capacity of the ER in promoting protein maturation and transport, the cell must deal with any proteins that remain unfolded or misfolded within the ER - the characteristic of the cell is known as ER stress [2-4]. It is believed that ER stress contributes to wide range of pathogenesis such as liver cirrhosis, diabetes, and Alzheimer's disease [5-8]. To help alleviate ER stress, controlled transcriptional response, called “Unfolded protein response (UPR)” composed of molecular chaperones promote the correct folding of nascent proteins in the ER and inhibit the aggregation of aberrant proteins. The key component of the UPR, glucose regulated protein 78 (GRP78), dissociates from three types of ER-localized transmembrane signal transducers to lead to their activation [9,10]. These transducers include two protein kinases IRE1 (inositol requiring kinase 1), and PERK (double stranded RNA-activated protein kinase-like ER kinase) and the transcription factor ATF6 (activating transcription factor 6) [3,9,10]. Together they promote correct folding, eliminate faulty protein, and restore ER homeostasis generally by decreasing translation initiation (mediated by IRE1α and ATF6), and selectively inhibiting translation of specific mRNAs (mediated by PERK) [3].
A recent study has shown that cells under ER stress activate autophagy (macroautophagy) [11-14]. Macroautophagy induced by stress involves the sequestration of cellular organelles by double-layered membranes called autophagosomes, which ultimately fuse with lysosomes and their contents are degraded by lysosomal hydrolases [15]. Autophagy is generally characterized by the presence of cytoplasmic vacuoles and autophagosomes, an increase in cleavage of LC3 (microtubule-associated protein 1 light chain 3), and a reduction in p62 (also known as SQSTM1) protein levels [16]. Under basal autophagy, long-lived proteins and damaged organelles are removed and the degradation products are released into the cytosol as intermediate metabolites. Like the UPR, autophagy is associated with both cell survival and cell death depending on the level and duration of autophagy [17].
Tunicamycin (Tun), a naturally occurring antibiotic, induces ER stress in cells by inhibiting the first step in the biosynthesis of N-linked glycans in the proteins resulting many misfolded proteins [18,19]. A few studies suggest that tunicamycin may work as a therapeutic drug to cancer cells as it sensitizes human colon and prostate cancer cells to apoptosis [20-23], however, at that dose it also produces cytotoxicity on normal cells. Here we examined a hypothesis that pharmacologically blocking of autophagy in the presence of mild ER stress would potentiate cell death of cancer cells. Our results showed that chloroquine (pharmacological blocker of autophagy) and mild dose of tunicamycin together caused synergistic cell death of prostate and breast cancer cells suggesting the combination treatment could be a promising strategy for cancer therapy.
Materials
Tunicamycin, chloroquine, mammalian cell lysis kit, Rabbit anti-actin antibody, and DMEM: F12 medium were purchased from Sigma-Aldrich. WST-1 cell viability kit and X-treme GENE HP DNA Transfection reagent were obtained from Roche. Gold antifade mounting medium, Nu PAGE Novex 4-12% Bis-Tris gels, goat anti-rabbit IgG-alexa Fluor 488, and Fetal Bovine Serum were from Invitrogen. Apodirect TUNEL assay kit was purchased from Millipore. Ultralow attachment tissue culture plate was from Nunc. DAPI and HRP chemiluminescence substrate were from Thermo Scientific. Anti-rabbit antibodies against LC3, caspase 3 and p62 were obtained from Cell signaling Technology. LC3-GFP construct was kind gift from Dr. Beth Levine (UT Southwestern, Texas).
Cell Culture
Androgen-independent metastatic prostate cancer cell line PC3 (ATCC, Manassas, VA), MDAMB436 (gift from Dr. Stuart Martin, University of Maryland), MDAMB231 (ATCC) were cultured in respective medium. Cell culture media, DMEM: F12 (1:1) (for PC3) and DMEM (for MDAMB231, and MDAMB436) were supplemented with 10% fetal bovine serum (FBS) (Quality Biologicals, Gaithersburg, MD), 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate (Sigma). All cells were cultured in the presence of 5% CO2 at 37oC.
Cell Viability and Cell Apoptosis
Cell viability was assessed on 96-well plate using WST-1 colorimetric assay according to the manufacturer’s instructions (Roche) [13,19]. TUNEL assay using Apodirect TUNEL assay kit (Millipore) for cell apoptosis was performed on a flow cytometer (FACSCanto, Becton Dickinson) using channel FL1. Also, to analyze whether the cell death was apoptosis the cells (5 x 106) were incubated with cleaved caspase 3 antibody (Cell Signaling Technology) at a ratio of 1:100 for 1 h at 37 °C followed by secondary goat anti-rabbit IgG alexa 488 antibodies (Invitrogen) (1: 1000) for 30 min at 37 °C. Finally, the samples were washed 3 times followed by flow cytometry at FL1 channel.
LC3-GFP Punctate Staining
PC3 cells stably expressing LC3-GFP were plated onto 2 well chamber slides (BD) and subjected to treatment with tunicamycin (5 mg/ml) for 24h. Cells were fixed with 3.7% formaldehyde (Sigma), examined by confocal microscopy (Olympus Model Olympus FV-1000) and digital images were obtained with CCD camera using Olympus FV-1000 viewer software. More than 5 puncta per cell was considered autophagy [13]. Cells were counted under in each field and 5 different fields were scored for statistical analysis.
Immunohistochemical Analyses
Immunohistochemical detection of cleaved caspase 3 and p62 using specific anti-caspase 3 and p62 antibody was performed on tumor (Cell Signaling Technology) as previously described [19,24,25]. In brief, sections were deparaffinized in xylene, dehydrated with absolute ethanol and then hydrated with distilled water. Samples were heated on a microwave oven in 1X Target Retrieval solution and then washed with phosphate buffered saline (PBS) for 5 min and incubated in 3% hydrogen peroxide to inhibit endogenous peroxidase. Samples were incubated with antibody (anti p62, or anti caspase 3) (1: 400) over night at 4 °C in a humidified chamber. After washing, the slides were incubated with secondary antibody conjugated with horse radish peroxidase followed by development with diaminobenzidine (DAB) substrate and counterstaining with Mayer’s hematoxylin. For statistical score, cells were counted under 20X on Axioplane 2 Zeiss microscope attached with cool CCD camera (Olympus model DP70) in each of four fields.
TUNEL Assay
DNA fragmentation in tumor tissue samples was done using TUNEL staining. Briefly, the tissue samples were deparaffinized followed by rehydration. The samples were permeabilized with 20 μg/ml Proteinase K (Gibco BRL) in 10 mM Tris-5 mM EDTA, pH 7.5 at 37 °C for 30 min. After washing with PBS, the samples were incubated in TUNEL staining solution using Apodirect TUNEL assay kit (Millipore) for 1 h at 37 °C in dark followed by counterstaining with DAPI. TUNEL positive cells were analyzed and counted under fluorescence microscope using green filter (for dUTP-FITC). TUNEL positive cells were counted under 20X in each field and 4 different fields were scored for statistical analysis.
Development of Breast Tumor Xenograft in Nude Mice
The work involving animals was approved by the Institutional Animal Care Committee of the University of Maryland. MDA MB231 cells (3 million) were mixed with 50% MatrigelTM (150 ml total volume) and injected subcutaneously (s.c.) in the hind region of each of Balb/c (nu/nu) female mice (six weeks old). One week later, the mice-bearing tumors were divided into four groups (7 mice per group) as follows. Group 1 received only CQ (60 mg/kg body weight) daily five times per week through intra peritoneal (i.p.) injection. Group 2 received only Tun (0.2 mg/kg body weight) once a week through ip. Group 3 received both CQ and Tun at same dose and same schedule as described for Group 1 and 2, while mice in the control group (Group 4) received the vehicle only. The tumor growth in each group was determined by measuring tumor with slide caliper 2-3 times each week. All animals were humanely euthanized after 16 days of treatment and tumors and tissues were processed for histological and immunohistochemical analyses.
Statistical Analysis
The statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Turkey-Kramer multiple comparisons (Graphpad Instat, version 3). The differences were considered significant when p˂0.05. Unless otherwise stated, all data are representative of three independent experiments with S.D. indicated by error bars. P values are determined by two-tailed student t-test and ˂0.05, ˂0.01 and ˂0.001 are indicated by one, two and three asterisks, respectively.
Tunicamycin Induces Autophagy
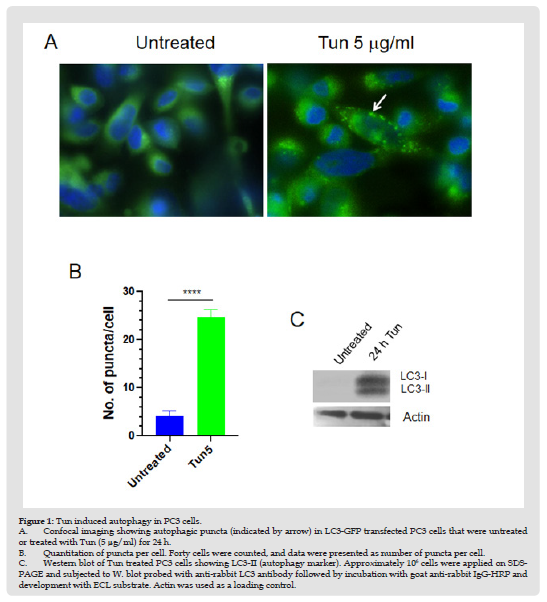
To search for new therapeutic strategies of hormone-refractory prostate cancer, we previously performed whole genome expression microarray analysis in Tun-treated PC3 cells and identified a few candidate genes that might be involved in the apoptotic process under ER stress [13]. When cell viability and cell apoptosis of PC3 cells were assessed at various doses of Tun over 96 hours, we observed no or negligible (<1%) apoptosis of cells during the first 48 h, but apoptosis was evident at 72h (about 15%) and 96h (more than 50%) [13]. To investigate if induction of autophagy was the reason for cell survival in the initial phase of Tun-treatment, we examined the formation of autophagic puncta after transfecting cells with LC3-GFP [13]. As previously shown [13], Tun treatment for 24h increased the number of puncta per cell by 5-fold more compared to DMSO treated cells suggesting an induction of autophagy (Figures 1A & 1B). Autophagic induction was further validated by Western blot analysis where increased expression of LC3-II (a cleaved form of LC3) was evident (Figure 1C). Taken together, Tun treatment of PC3 cells for 24 h didn’t induce trigger apoptosis due to induction of autophagy.
Figure 1 Tun induced autophagy in PC3 cells. A. Confocal imaging showing autophagic puncta (indicated by arrow) in LC3-GFP transfected PC3 cells that were untreated or treated with Tun (5 μg/ml) for 24 h. B. Quantitation of puncta per cell. Forty cells were counted, and data were presented as number of puncta per cell. C. Western blot of Tun treated PC3 cells showing LC3-II (autophagy marker). Approximately 106 cells were applied on SDSPAGE and subjected to W. blot probed with anti-rabbit LC3 antibody followed by incubation with goat anti-rabbit IgG-HRP and development with ECL substrate. Actin was used as a loading control.
Inhibition of Autophagy Potentiates Cytotoxic Effect of Tunicamycin (Tun)
To assess the importance of initial autophagy activation during ER stress, PC3 cells were treated with autophagy blocker chloroquine (CQ) in the presence of Tun and viability of cells was analysed by WST-1. CQ raises the pH and reduces autophagic degradation by blocking fusion of autophagic vacuoles with lysosomes. As shown in Figure 2A, PC3 cells showed 28% inhibition of cell viability with Tun (5 mg/ml) treatment for 72 h, while CQ (50 mg/ml) treatment alone for 72h exerted inhibition of cell viability by 10%. Interestingly, Tun plus CQ combination amplified inhibition of cell viability to almost 54%, which is greater than the additive suggesting a synergistic effect (Figure 2A).
Figure 2 Analysis of cytotoxic effect of Tun and CQ on PC3, MDA MB436, and MDA MB231 cells. A. Synergistic cell death of PC3 cells in the presence of CQ and Tun. Cells were treated with either Tun (5 μg/ml) or CQ (50 μg/ml) or in combination for 72 h and cell viability was measured by WST-1 staining. B. Synergistic cell death of MDA MB 436 cells in the presence of CQ and Tun. Cells were treated with either Tun (1 μg/ml) or CQ (50 μg/ml) or in combination for 72 h and cell viability was measured by WST-1 staining. C. TUNEL assay. Cells (PC3, MDA MB436, and MDA MB231) were treated with Tun (5 μg/ml) alone, CQ (50 μg/ml) alone, or in combination for 72 h (PC3) and 48 h (MDA MB436 and MDA MB231) and TUNEL assay was performed on a flow cytometer. The number inside the flow diagram represents the percentage of TUNEL positive cells.
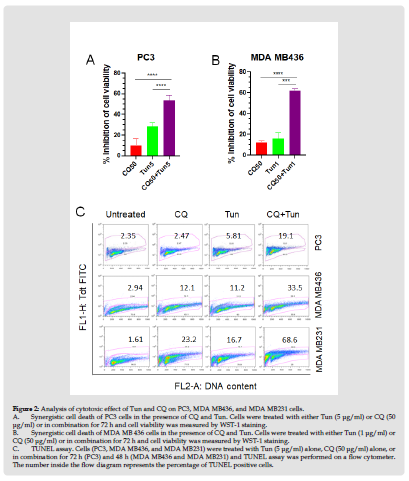
The effect of Tun+CQ was also evaluated on a triple negative breast cancer cell line, MDA MB436. Treatment of MDAMB436 cells with CQ alone for 72 h caused inhibition of cell viability by 12%, while MDA MB436 cells in the presence of Tun (1 mg/ml) showed 16% inhibition of cell viability. However, consistent with the PC3 cells, combination (CQ plus Tun) treatment of MDA MB436 cells caused synergistic cell death to 62% (Figure 2B). To confirm if the inhibition of cell viability was a result of apoptosis, TUNEL assay of both PC3 and MDA MB436 cells was performed in the presence of CQ (50 mg/ml), Tun (5 mg/ml), and CQ+Tun for either 72 hr (PC3) or 48 h (MDA MB436). Co-treatment of PC3 cells with CQ and Tun caused a synergistic apoptosis by 19.1% (~2.5% with CQ alone and ~5.8% with Tun alone; Figure 2C). Similarly, treatment with CQ and Tun combination exerted synergistic apoptosis of MDA-MB436 cells by 33.5% (~12% with CQ alone and ~11% with Tun alone; Figure 2C). The synergistic effect of CQ and Tun combination was further confirmed on another aggressive triple negative breast cancer cell line, MDA MB231. As expected, co-treatment of MDA MB231 cells with CQ (50 mg/ml) and Tun (5 mg/ml) for 48 h caused a synergistic apoptosis by 68.6% (~23% with CQ alone and ~17% with Tun alone; Figure 2C).
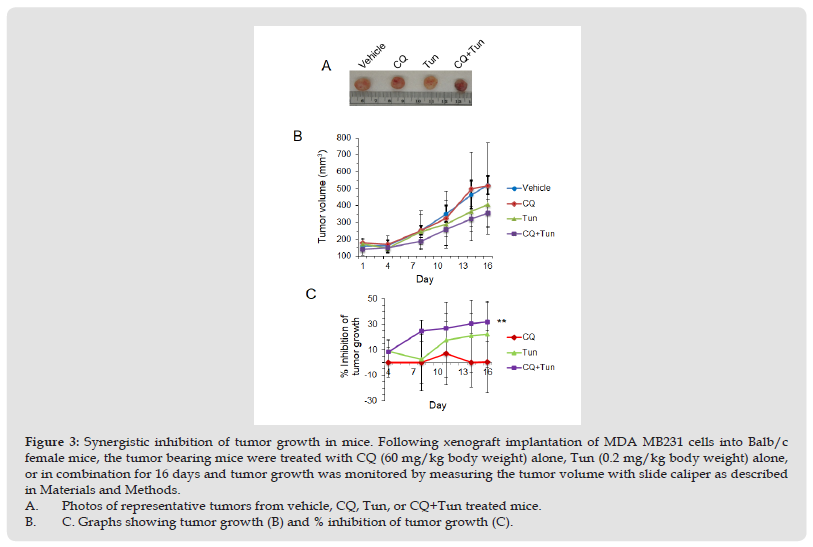
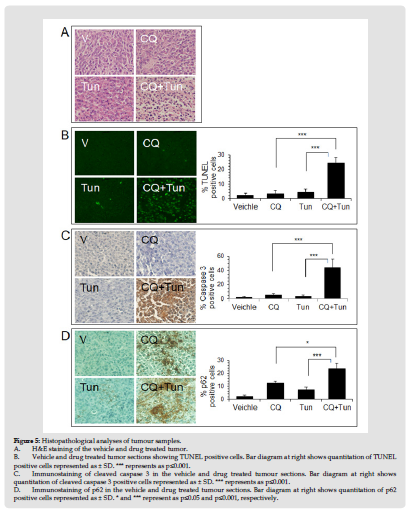
CQ potentiates effects of Tun in mice model of tumor. Lastly, we sought to investigate whether targeting autophagy and ER stress could impact tumor growth in vivo. MDA-MB231 cells were chosen for the xenograft implantation as the highest number of cells were found dead with the CQ+Tun combination treatment. Once the tumor reached the volume of 100 mm3, mice were treated with CQ (60 mg/kg body weight, daily i.p.) alone, Tun (0.2 mg/kg, once a week) alone, and CQ+Tun. While CQ alone had no significant effect on tumor growth after 16 days, Tun alone inhibited tumor growth by 22% (Figures 3A-3C). Consistent with the in vitro data above, a combination of CQ and Tun had a synergistic inhibitory effect on tumor growth (32% inhibition compared to the vehicle, p<0.01, Figures 3A-3C). The combination drug didn’t cause any significant loss of body weight during therapy (Figure 4A). Moreover, histology of liver (Figure 4B) and kidney (Figure 4C) from the treated animals seem normal suggesting no toxicity of the drug. Histology of the drug treated tumors was performed using H&E staining and the CQ+Tun treated tumors seemed to have different morphology compared to either vehicle or CQ treated tumor (Figure 5A). Immunohistochemical analyse of the tumor samples were analyzed for any TUNEL positive cells, caspase 3 activation, and p62 expression.
Figure 3 Synergistic inhibition of tumor growth in mice. Following xenograft implantation of MDA MB231 cells into Balb/c female mice, the tumor bearing mice were treated with CQ (60 mg/kg body weight) alone, Tun (0.2 mg/kg body weight) alone, or in combination for 16 days and tumor growth was monitored by measuring the tumor volume with slide caliper as described in Materials and Methods. A. Photos of representative tumors from vehicle, CQ, Tun, or CQ+Tun treated mice. B. C. Graphs showing tumor growth (B) and % inhibition of tumor growth (C).
TUNEL assay with tumor tissues showed that CQ+Tun treatment significantly (p<0.001) induced apoptosis (24.5%) compared to either CQ or Tun treatment (range 3.5-4.5%) (Figure 5B). The number of cleaved caspase 3 positive cells (44.5%) was also significantly (p<0.001) higher in the CQ+Tun treated tumor compared to either CQ or Tun treatment (Figure 5C). Lastly, the expression of p62 was examined. p62 protein accumulates in cells when autophagy gets inhibited. As expected, CQ treated tumor showed increased expression of p62 (almost 2-fold) compared to the Tun treated tumor (Figure 5D). However, p62 staining was stronger in Tun+CQ treated tumor compared to either CQ (p<0.05) or Tun (p<0.001) alone.
Recently, endoplasmic reticulum (ER) has emerged as a prominent target for the development of chemotherapeutics against diverse diseases including diabetes and cancer [26]. Tunicamycin (Tun), a nucleoside antibiotic, inhibits the first step in the biosynthesis of N-linked oligosaccharides in cells, resulting in the ER stress induction and apoptotic cell death by unfolded protein response (UPR) in certain cancer cells [27]. However, at regular dose, which kill different cancer cells, Tun showed severe nephrotoxicity, hepatotoxicity and neuro toxicity in mice and rat model [28-30]. Because of its profound toxicity on normal physiology use of Tun in cancer treatment is restricted.
If proteotoxic stress in the cytosol exceeds the capacity of the ubiquitin proteasome system to ubiquitinated proteins, autophagy can compensate for protein degradation and contribute to cell survival [31]. Hence, inhibition of autophagy could amplify cellular stress dependent death response. Interestingly, we found robust induction of autophagy at very low (1 and 5 μg/ml) dose of Tun in prostate (Figures 1A & 1B) and breast cancer cells (not shown). At this dose Tun showed little cytostatic effect on both the cancer cells (Figures 2A-2C). However, combination of Tun+ CQ markedly influenced Tun induced cytotoxicity on both the cancer cells in vitro (Figures 2A-2C) and in the mouse model of tumor (Figure 3). It is important to mention that CQ alone also had little effect on cytotoxicity of tumor cells both in cell culture model and animal model. Inhibition of autophagy with CQ stalled degradation of unfolded toxic proteins as signified by accumulation of p62 (Figure 5), binds to ubiquitinated proteins followed by degradation through autophagy. Sequestration of ubiquitinated-unfolded proteins could induce intracellular ROS generation thus exponentially aggravated ER stress dependent death response in tumor cells cell (Figures 2 & 3).
Figure 4 Body weight and histology of tumor tissues. A. Body weight of the vehicle and drug treated mice during the therapy. B. C. H&E staining showing histology of liver (B) and kidney (C) from the vehicle and the drug treated mice.
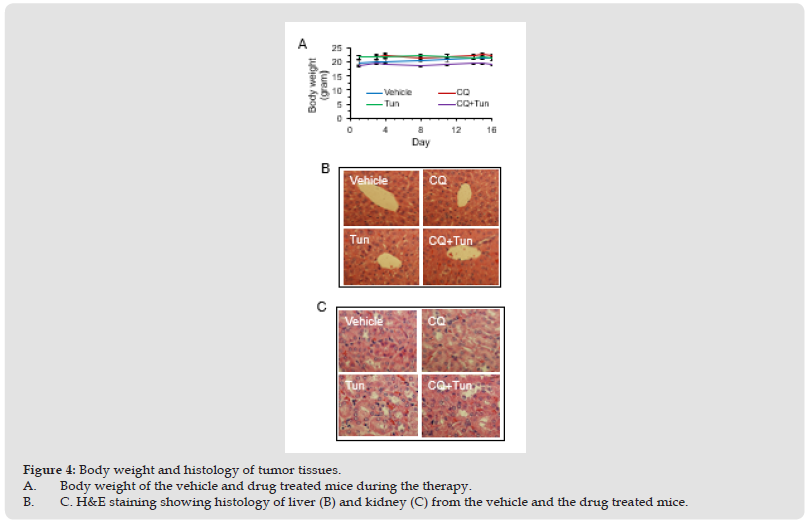
Figure 5 Histopathological analyses of tumour samples. A. H&E staining of the vehicle and drug treated tumor. B. Vehicle and drug treated tumor sections showing TUNEL positive cells. Bar diagram at right shows quantitation of TUNEL positive cells represented as ± SD. *** represents as p≤0.001. C. Immunostaining of cleaved caspase 3 in the vehicle and drug treated tumour sections. Bar diagram at right shows quantitation of cleaved caspase 3 positive cells represented as ± SD. *** represents as p≤0.001. D. Immunostaining of p62 in the vehicle and drug treated tumour sections. Bar diagram at right shows quantitation of p62 positive cells represented as ± SD. * and *** represent as p≤0.05 and p≤0.001, respectively.
The Tun dose up to 1 mg/kg body weight was previously used in a few xenograft mice models of cancer and animals neither exhibited weight loss nor showed any sign of abnormal behavior [19]. The Tun concentration (0.2 mg/kg body weight) used in our animal experiment was at 5-times less and not expected to exert any toxicity in mice. In fact, the histology of liver and kidney from the Tun treated (either alone or in combination with CQ) appeared normal (Figure 4B). The combination therapy of an aggressive triple negative metastatic breast cancer MDA MB231 using low dose of Tun and CQ produced a modest result (32% inhibition of tumor growth compared to the vehicle treated animals), nonetheless, it seems promising considering the short duration of the therapy (16 days). However, further studies of this combination drug in various cancer models are needed to confirm its therapeutic potential.
This study was supported by the University of Maryland start-up fund, and in part by the NIH Grants CA133935 and CA141970 to H.A.


