Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Alimova L N and Abdullabekova V N*
Received: December 02, 2022; Published: December 16, 2022
*Corresponding author: Abdullabekova V N, Tashkent pharmaceutical institute, department chair of pharmaceutical chemistry, Uzbekistan
DOI: 10.26717/BJSTR.2022.47.007539
Researches on studying of component are conducted of liquid extract. That liquid extract contains such bonds as flavonoids, terpenoids and glycyrrhizic acid. The unified analysis techniques on the BAV main group in a dosage form with use of UV – the spectophotometry, a method of a chromatography (HPLC, TLC) and a chromatomass- spectophotometry [1]. This methods are allowed to estimate objectively quality of drug developed. We got the amount of flavonoids 0,98%, glycyrrhizic acid - on average 0,34% and terpenic bonds of-0,807% [2-4].
Keywords: Spectophotometry; Chromatography; Chromatomass-Spectophotometery; Flavonoids; Glycyrrhizic Acid; Terpenic Bonds
The most important part of the public policy of the Republic of Uzbekistan in the field of health care is the provision of the population and the saturation of the domestic market with high-quality, effective and safe domestic medicinal products of plant origin [5]. At the same time, the main focus is on those types of products, the implementation of which does not require long-term investments of time and money [6]. The provision of such drugs is expedient and economically profitable to implement through the development of phytochemical productions. Therefore, research aimed at creating and standardizing as well as quality control of herbal medicinal products is currently relevant [7-10]. The main step in the standardization of medicinal products of plant origin is the study of the main groups of biologically active substances (BAV), which determine their therapeutic activity. The purpose of this research is to study the main groups of biologically active substances of the liquid extract obtained from the local raw material of licorice root, sage leaves and nettle for its standardization and implementation in medical practice as an anti-inflammatory and antipyretic agent [11]. Preclinical studies of the liquid extract have shown that it has pronounced anti-inflammatory, expectorant and hemostatic effects and does not cause allergies [1]. Preliminary studies on the study of BAB showed that the extract contains flavonoids, terpenoids, glycyrrhizic acid [12].
The study of flavonoids of the liquid extract was carried out by the thin-layer chromatography (TLC) method. “Silufol UV-254” (Czech Republic) and “Sorbfil” ПЭТФ (10х10) УФ-254 (silica gel СТХ-1БЭ) plates were used, as solvent system were used: chloroform - methanol (19:1); chloroform–methanol (9:1); chloroform– methanol (4:1); н – butanol-pyridine – water (6:4:3), alcohol-toluene (1:3), alcohol-toluene (2:3). The best separation of flavonoid compounds was observed when using the ethyl alcohol-toluene (2:3) system [13-15]. Individual flavonoids were identified: rutin Rf=0.45 and quercetin Rf=0.78. When developing chromatograms, a 5% alcohol solution of aluminum chloride and a 10% solution of sodium hydroxide were used as a detector. At the same time, the spots acquire a bright yellow color and yellow fluorescence in the visible area of UV light [16]. The quantitative content of the amount of flavonoids in terms of rutin was determined by the UV-spectrophotometric method [64] on the spectrophotometer brand “Lambola 16” Perkin-Elmer, “Spectroscopy system 8456E” (для снятия УФ-спектра) [17]. They measured the optical density of the investigated solution and the РСО rutin solution at a wavelength of 408 nm in a cuvette with a layer thickness of 10 mm. The obtained results are presented in Figures 1 & 2. As can be seen from the presented figure, the wavelengths of the test solution are 400 nm and 408 nm - rutin alcohol solution.
Quantitative content of the sum of flavonoids in % per rutin was calculated according to the following formula:
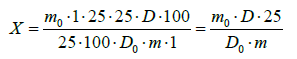
where: D0 is the optical density of the PCO solution of rutin;
D0- optical density of the test solution;
m0- mass of RCO rutin, in grams;
m -mass of the drug, in grams;
The results of the studies and metrological characteristics of the UV spectrophotometric method of analysis are presented in Table 1. As can be seen from Table 1, the content of the sum of flavonoids is on average 0.98%, and the error of the method is ±1.65%. The study of the terpenoid composition of the liquid extract was carried out by gas chromatography-mass spectrophotometry [18]. Chromatography was carried out in the Instrument -HP GC/MS 6890/5973 with column HP-FFAP Polyethylene Glycol TPA capillary 50.0m x 200μkm x 0.3μkm nominal under the following conditions: mobile phase –He (helium); the speed of the mobile phase in the column 1.0 ml/min; the volume of the injected sample is 1 μl; in Splitless mode; evaporation chamber temperature - 200◦С; detector temperature 230°C; Aux-260◦C; the column temperature is gradually increased from 70°C at a rate of 10°C/min to 180°C over 10 min. Total analysis time 27 min. To identify substances, the Willey- 2 software library is used. The results of the analysis are shown in Figure 3. Analysis of the presented chromatograms showed that the liquid extract contains such terpenoid compounds as alpha-terpineol (0.054%), alpha-thujone (0.13%), 1,8-cineole (0.089%), camphor (0.229%) and borneol ( 0.305%) [19].
Identification and quantification of glycyrrhizic acid was carried out by HPLC. Chromatography was carried out under the following conditions: column: Luna C18, 5 μm (150x4.6 mm) or similar; mobile phase: acetonitrile-water-glacial acetic acid (614:380:6); flow rate: 1.0 ml/min; injection volume: 20 μl; wavelength: 254 nm; run time: 25 minutes; holding time: about 13 minutes. Analysis of the chromatograms presented in Figures 4 & 5 showed that the peak with time 13.8 corresponds to glycyrrhizic acid. The content of glycyrrhizic acid in the preparation as a percentage is calculated by the following formula:
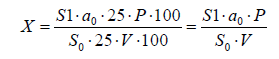
where S0 is the area of the peak of glycyrrhizic acid in the chromatogram of the standard solution; S1 is the area of the peak of glycyrrhizic acid on the chromatogram of the test solution; a0 is the mass of a sample of the RSO of glycyrrhizic acid, mg; V is the volume of the drug taken for analysis, ml; P is the content of the main substance in the RSO of glycyrrhizic acid, in percent. The results of the studies and metrological characteristics of the HPLC analysis method are presented in Table 2. The results of the studies showed that the quantitative content of glycyrrhizic acid in the liquid extract is on average 0.34%, and the error of the HPLC analysis procedure (at f=4; t=2.78; P=95%) is ±2.58%. Thus, the results of the conducted studies on the study of the component composition of the liquid extract showed that the liquid extract contains such biologically active substances as flavonoids, terpenoids and glycyrrhizic acid. Unified analysis methods have been developed for the main group of biologically active substances in the dosage form using UV spectrophotometry, chromatography (HPLC, TLC) and chromatography- mass spectrophotometry (HM/SF), which allow to objectively assess its quality. At the same time, the content of the total flavonoids is 0.98%, glycerrisic acid is on average 0.34% and terpene compounds - 0.807%.
Table 2: Results of quantitative determination of glycyrrhizic acid in liquid extract and metrological characteristics of the analysis technique (at f=4; t=2.78; P=95%).
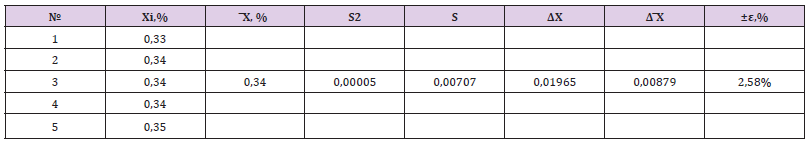
The obtained results of the conducted studies served as the basis for the development of the VFS project for the liquid extract. Based on the above data, the norms for the quantitative content of these substances in the liquid extract are established: the content of the total flavonoids must be at least 0.9%, the content of glycyrrhizic acid must be at least 0.3%.


