Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Muhammad Shahbaz1*, Shagufta Zubair2, Muhammad Akhlaq3 and Abdul Moiz4
Received: December 04, 2022; Published: December 16, 2022
*Corresponding author: M Shahbaz, Mawarid Food Company, Al Wizarat, Riyadh 11544, Saudi Arabia
DOI: 10.26717/BJSTR.2022.47.007536
The consumption and sale of fresh-cut products and salads have been growing tremendously in the present era. Therefore, the microbial safety of such products is of great concern. In the current study, a survey of general microbiological safety of fresh-cut produce and salads at quick-service restaurants (QSR) was undertaken across the Kingdom of Saudi Arabia. These findings were compared with microbiological criteria for foodstuffs by Saudi standards, metrology, and quality organization SASO-GSO-1016. Of the 82 samples of fresh-cut produce, 7% of samples were found to be unsatisfactory or beyond the acceptable limits. TPC count was unsatisfactory at 22%, coliform at 48%, and Staphylococcus aureus at 4%. For 108 samples for fresh salads, 11% of samples were found to be unsatisfactory or beyond the acceptable limits, 13%, 27%, 4%, and 27% of samples showed an unsatisfactory range of TPC, coliforms, S. aureus, and Escherichia coli, respectively. The fresh-cut produce and salads were microbiologically safe in the central region compared to the eastern region followed by the western region. The relatively higher count was found in green pepper, mixed vegetables, and lettuce followed by fresh-cut onions and coleslaw salads. No Salmonella was detected in both fresh-cut produce or salads. The restaurants should be more stringent in their processing to ensure consumer safety. Washing and sanitization of produce is the only way to reduce the diffusion of food borne pathogens.
Keywords: Food safety; Fresh-Cut Produce; Salads; Food Borne Pathogens; Microbiological Safety
Any physically modified form of fresh vegetables but remaining fresh is called freshly cut salad and vegetables. The use of fresh-cut salads and vegetables is healthy and is a growing category of foods with limited value-added. and fresh in taste and is an increasingly growing category of foods with limited value-added [1]. Despite a few examples, microbes are present across the world, including sterilized surfaces according to Balali, Yar, Afua Dela, & Adjei-Kusi, (2020) [2]. These include normal nonpathogenic flora, which contributes to a greater proportion, and few other pathogenic species. A natural layer of the waxy cuticle is present on the outer protective epidermis of fruits and vegetables [3]. The protective layer is the natural barrier that prevents the growth on the undamaged surface of fruits and vegetables. In recent years, the consumption and sale of fresh-cut produce have expanded significantly. So, the microbiological safety and quality of such goods are of great concern. In addition, fresh-cut goods are more vulnerable to the rapid growth of micro-organisms. Humans, animals, insects and soils often come in contact with vegetables from farm to table, thus contaminating food pathogens and microorganisms [4]. Complex microflora naturally contaminates fresh-cut foods due to a variety of factors, including the farm environment, post-harvest handling, and processing. Complex microflora naturally contaminates fresh-cut foods due to a variety of factors, i.e. farm conditions, harvesting equipment, products handling, processing, manufacturing, transport, and distribution of the product [4-6].
According to previous studies, the use of freshly cut vegetables and salads may present a risk of pathogenic infection [7,8]. Recently, several outbreaks are reported from the consumption of contaminated fresh produce infected with Salmonella and E. coli. Previously, a regional salmonella outbreak occurred in Finland during 2008, the salmonella was reported as a responsible pathogen in the iceberg lettuce [8,9] found 14 samples of vegetables (fresh and cooked) contaminated with S. aureus in his survey conducted on restaurants. In literature, the contamination of Intestinal parasites was also reported in various raw vegetables collected from different forms and local supermarkets in the United Arab Emirates [10]. Therefore, this study aimed to evaluate the safety status of various freshly cut salads and vegetables in various restaurants located in different regions of the Kingdom of Saudi Arabia, i.e., eastern region (Dammam Alkhobar), central region (Riyadh) and western region (Makkah and Madinah).
Samples Collection
Quality assurance department of Mawarid Food Company Saudi Arabia coordinated the survey and developed the survey protocol that was shared with an accredited food laboratory (ALS Arabia) for sample collection and analysis. The samples of fresh-cut produce and salads (190 samples collectively) were collected randomly from the restaurants located in three regions central (Riyadh), eastern (Dammam, AL Khobar), and western (Makkah and Madinah) of the kingdom of Saudi Arabia from March 2017 to June 2019. Fresh-cut produce comprised of 82 samples of fresh- cut onions, green pepper and mixed vegetables. 108 samples of four types of salads comprised of coleslaw, lettuce, cut tomatoes, and cut potatoes. All the samples were collected from the display area and salad bars of various quick service restaurants which were maintained at a cold temperature of 1- 5°C and all samples were within time and temperature control as mentioned on the labels. All samples were collected by trained samplers by using sanitized salad spoodles and tongues and were placed in sterile plastic bags and transported properly to the lab in ice boxes. Samples that were not at cold temperatures were discarded before analysis.
Microbiological Analysis
Aerobic Colony Count: The plate count agar (Oxoid CM0325, UK) was used for the testing of aerobic colony count. The phosphate- buffered saline was used for sample dilution. First dilution was prepared by taking one gram of the sample in a test tube containing 9ml of saline solution. Now this first dilution was used for the preparation of further serial dilutions in buffered saline solution. Now the spread plate method was used for the inoculation of 1ml sample dilution in aerobic plate count agar. Now the plates were placed and incubated for 24-72 hours at 30-32℃. The colonies were counted by using Whitely Automated Spiral Platers (Don Whitley, Shipley, UK) by following the manufacturer’s instructions.
Coliforms and E. Coli O157: The method was based on ISO 4832:2006 and USFDA BAM 4. A test portion (1 mL) or serial dilution of 1:10 sample (10 g + 90 mL) was transferred into the plate and poured with 15-20 mL violet, red bile agar (VRBA) (M174). Allowed the VRBA to solidify and afterward overlay with an additional 5 mL of VRBA. Plates were inverted and incubated at 37±1 °C for 22 to 26 h for enumeration of coliforms. The remaining plates incubated at 37±1 °C for 3-5 h and then transfer the plates to incubate further at 44±1 °C for 18 to 24 h. The number of colonies were enumerated and further proceeded for indole production to confirm the presence of E. coli O157 [11].
Staphylococcus Aureus: The method was based on USFDA BAM 12. 1ml suspension of the sample was transferred aseptically and distributed equitably (0.4ml, 0.3ml and 0.3ml) to three plates containing medium (Baird-parker-M17). The inoculum was spread over the entire surface of agar plate with the aid of sterile streaking rod (bent glass). Now the agar plates were held upright for about 10 minutes until the plates were dried by properly absorbing the inoculum, and then plates were incubated in inverted position for 45- 48 h at 35-37°C. For the coagulase test, suspected Staphylococcus aureus colonies were transferred to small tubes containing Brain heart infusion (BHI) broth (M24). Only when the clot stayed firm with the test tube even after the test tube was twisted and rotated, the clot was deemed positive for S. aureus [11].
Salmonella: Based upon USFDA BAM 5, the initial enrichment 1:10 (25g + 225ml) was prepared in peptone water (M192) and then incubated at 36℃ +2℃ for 18 to 24 hours. Inoculated the 0.1ml of pre- enriched BPW into 10ml Rappaport-Vassiliadis medium (RV) (M132) and then incubated for 24 + 3 hours at 41.5℃ + 1℃. In parallel, 1ml of pre-enriched BPW was inoculated into 10ml Muller- Kauffmann Tetrathionate-Novobiocin Broth (MKTTn) CM 1048 and incubated at 37±1℃ for 24h. Using 10μl loop, streak from each broth (RV and MKTTn) was used to inoculate the selectively enriched broth Xylose lysine deoxycholate agar (XLD) (M179) and chromogenic BSA, and then incubated for 24 hours at 37℃. The results were confirmed with the typical poly O and poly H antiserum as well as with the biochemical gallery API20E [11].
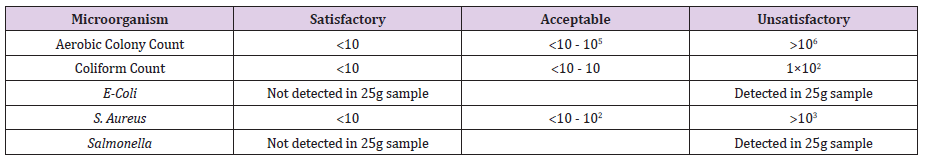
Statistical Analysis: The data was analyzed through Minitab-16 using two-way ANOVA and Pearson’s correlation. Tukey’s multiple comparison test was used to compare the means at probability of 0.05. The data was also compared with the Guideline of Saudi Standards, Metrology, and Quality Organization (SASO-GSO-1016) for microbiological criteria of foodstuffs (Table 1).
Table 1. Guideline of Saudi Standards, Metrology, and Quality Organization (SASO-GSO-1016) for microbiological criteria of foodstuffs.
Fresh-cut goods are ready-to-eat products that provide a 100% functional product compared to whole fresh products, which is why processing is often required [1]. The consumption of contaminated vegetables and salads were linked to foodborne outbreaks in different countries. In Latin America (2000-2010), USA (1998–2007), and New Zealand (2012), the outbreaks due to contaminated vegetable were 4.4%, 33%, and 26.6%, respectively [12-14]. The results of the fresh-cut vegetables collected from different three restaurants are given in Table 2 and Figures 1 & 2. According to results, the restaurant located in the west region showed a higher total microbial count (62.5 log cfu/g), while central region showed the least total microbial count (48 log cfu/g). The total count of TPC (38.2 log cfu/g) and coliform (19.2 log cfu/g) were also higher in the west region, whereas S. aureus was recorded to be higher in the east region (6.0 log cfu/g). During a comparative evaluation of vegetables of these three regions, the higher total microbial count was noted in green pepper (58.0 log cfu/g), while the lowest in fresh-cut onion (51.1 log cfu/g). The TPC count was also higher in green pepper (40.5 log cfu/g) followed by fresh-cut onion (36.0 log cfu/g), whereas the count of coliforms and S. aureus was higher in mixed vegetables (7.4 log cfu/g) followed by green pepper (4.1 log cfu/g). In the current survey, only TPC was differed significantly (p < 0.05), whereas other microbes in fresh-cut vegetables and restaurants were non-significant. E. coli was detected in 30% of the samples, 7% samples of which were unsatisfactory or above the acceptable limit. Varied quantity of the samples was also found unsatisfactory in TPC (22 %), coliforms (48%), and S. aureus (4%). Salmonella was not detected in any sample. The results of the fresh-cut salad are portrayed in Table 3 and Figures 3 & 4.
Table 2.Microbial load (log cfu/g) of different fresh-cut produce collected from various restaurants located in three different regions of the Kingdom of Saudi Arabia and compared with SASO-GSO 1016 standards.
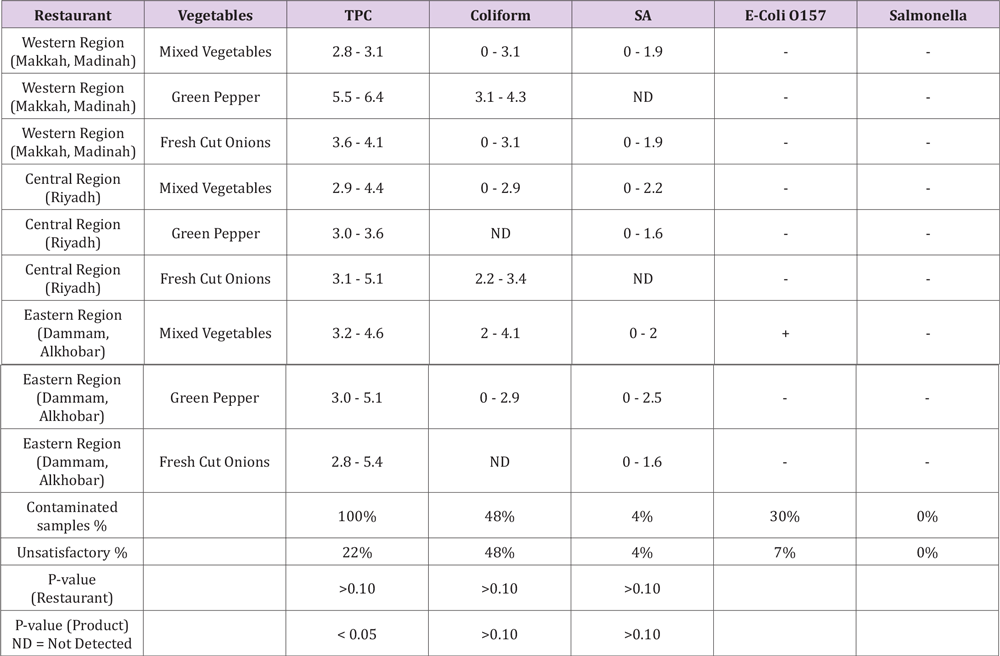
Table 3.Microbial load (log cfu/g) of different fresh-cut salads collected from various restaurants located in three different regions of the Kingdom of Saudi Arabia and compared with country standards.
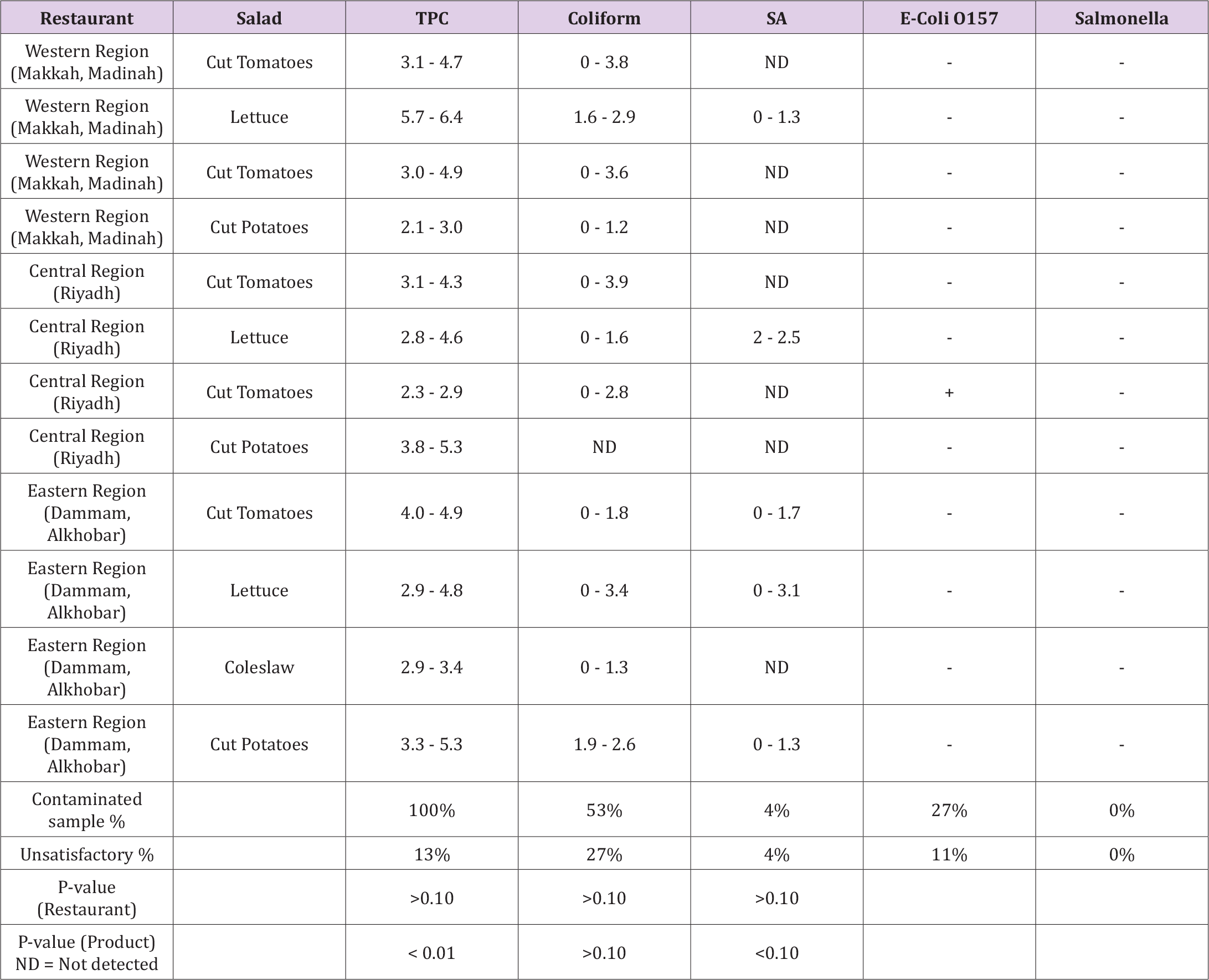
According to results, the highest total microbial count was found in fresh-cut salads collected from the restaurant in the west (87.5 log cfu/g), whereas the least count was found in the restaurant in central (76.6 log cfu/g). The total microbial count of TPC (58.6 log cfu/g) and coliform (21.0 log cfu/g) were higher in the west region, whereas S. aureus was higher in the east region (10.6 log cfu/g). Restaurants in the central region showed lower microbial count (collectively as well as individually). Salmonella was not detected in any sample, while E. coli was found in 27% of the samples, of which 11% were unsatisfactory and above the acceptable limit. The TPC was found in the range of 2.1-6.4 log CFU/g, with 13% samples above the acceptable limit. Coliforms ranged from 0.0 to 3.9 log CFU/g with 27% unsatisfactory samples. S. aureus was detected in the range of 0.0–3.0 log CFU/g, with 4% of unsatisfactory samples. Among different fresh-cut salads, the lettuce was found higher in the total microbial count, while the lower count was detected in coleslaw. Environmental factors may have a major impact on bacterial populations. The presence of free moisture on the surface of the substance may encourage the survival and growth of bacterial populations [6]. Although the full elimination of microbial foodborne pathogens is not possible, there are several available methods for maintaining food safety and reducing pathogens in fresh produce to an acceptable limit, including biological, chemical, and physical methods [15]. Like the present study, S. aureus and E. coli were also reported previously in several vegetable dishes of various restaurants. E. coli was found in 6.6% of samples, and contrary to the present study, 0.7% samples have also defected with salmonella. Freshly cut salads were sanitized and cleaned during the process, according to researchers, the improper cleaning and inadequate hygienic handling could contaminate the product [8]. Similar to this research, the literature also identified aerobic mesophilic microorganisms as the most persuasive microbial counts in minimally processed vegetables commercialized in Brazil (102 to 107 CFU/g) [16].
Similar findings were also reported by Faour-Klingbeil, Todd, & Kuri, (2016) [17] who found TPC in the range of 2.90 to 7.38 log CFU/g. They also found 17% defected samples above the maximum limit (>107 CFU/g), while in present report, the unsatisfactory samples were 22% (vegetables) and 13% (salad) (>106 CFU/g) (Table 1). E. coli and S. aureus were detected in 31.3% and 41.5% of samples, respectively. In contrary, the Salmonella was also detected in 0.9% samples. Previously, several foods born outbreak related to the use of freshly cut produce were reported in Brazil (2008– 2014). The causing agent for most of the outbreaks was salmonella, S. aureus, E. coli, and B. cereus with 30%, 23.3%, 10%, and 6.6% outbreaks, respectively [18]. Researchers are encouraging the use of freshly cut vegetables and salads but recommended substantial steps to ensure the safety status of food products before [2]. In the processing of freshly cut goods, different steps and methods are discussed previously, including sorting, cleaning, washing, cutting/ peeling, coring, slicing, shredding and packaging, depending on the type of vegetables and salad [1]. During these processing steps in the supply chain of fresh-cut supply, there are several points at which the microbial contamination can occur in each of these phases [19]. Several reports detected an increase in the microbial count during shredding, rinsing, slicing, centrifugation, processing machines and packaging of fresh-cut products [15,20,21]. Bacteria can adhere to and propagate on the food surface during harvesting, transportation, and storage. Therefore, they should be washed properly with saline solution or chlorinated water before consumption. Contamination due to washing water, however, can lead to an increase in microbial load on the product surface and therefore on the final fresh products. In the literature, the last step (washing) showed a substantial decrease in microbial counts [15,22], but in contrast, Qadri et al. (2015) found an increase. In the supply chain of freshly cut produce, the washing and sanitation of goods is considered as a main step in reducing the pathogenic microorganisms [1,23,24].
Handling and storage are considered as probable causes for most of the outbreaks related to vegetables. Cross-contamination is one of the contributing factors for the outbreak associated with fruit and vegetable consumption. The role of food handlers is very critical, as they are often involved in processes. In several studies, a potential source of many outbreaks was the food handler infected with the microorganism. In one outbreak, the chef of a restaurant in England and Wales became ill during work and suddenly contaminated the salad by vomiting over it during salad cutting. Instead of dumping the contaminated salad, he just rinsed and washed the salad in cold water and served it to the customer. In another E. coli outbreak, the staff at the restaurant stored the freshly cut produce in plastic containers that had previously been used to store raw beef. These containers were rinsed out only before being used again for salad vegetables. The packaging of fresh-cut products is found to be the main factor affecting microbial load and safety of produce. In the most part, the freshly cut goods are preserved and refrigerated after packing under modified atmospheric conditions. Any mishandling can lead to the development of pathogenic microorganisms by providing favorable conditions and time duration [9,18,25-28].
Fresh-cut produce and salads are very nutritious and widespread in consumption with the focus of modern consumers and lifestyle. Previously, several microbial outbreaks were linked to the consumption of contaminated foodstuff and were having major public health concerns. In the present study, 190 samples of freshcut produce from three different regions of the Kingdom of Saudi Arabia were collected and analyzed for TPC, coliforms, S. aureus, E. coli, and Salmonella. Salmonella was not detected in any sample, while 4% of the fresh-cut samples (vegetables and salads, both) were not satisfactory due to S. aureus. E. coli exceeded the legal limits in 7% and 11% of samples of fresh-cut vegetables and salads, respectively. Restaurants should make sure washing of vegetables with potable water and sanitize these with approved sanitizers with recommended contact time to make it safe for consumption.
This work was financially supported by the Mawarid Food Company, Riyadh Kingdom of Saudi Arabia under Lorenzo quality assurance program.
The listed author(s) declare no conflict of interest in any capacity, including competing or financial.


