Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Leena Grace Beslin*
Received: November 09, 2022; Published: December 14, 2022
*Corresponding author: Leena Grace Beslin, Department of Zoology, Nesamony Memorial Christian College (Affiliated to Manonmaniam Sundaranar University), Marthandam-629165, Kanyakumari District, Tamil Nadu, India
DOI: 10.26717/BJSTR.2022.47.007530
Bacterial infections cause high rate of mortality in human populations as well as aquatic organisms. Some of the bacteria cause highly dangerous diseases in humans and animals. Some of the antibiotics or chemicals are used to prevent bacterial infection. Some naturally available products are used to inhibit some dangerous disease-causing bacteria. The purpose of the current study was to evaluate and compare the antibacterial activity and the biochemical composition of cuttlebone. In the present study, the samples were collected from Leepuram coastal area, which is located in Kanyakumari District, Tamil Nadu. The samples were extracted by using different solvents like water, ethanol, methanol, acetic acid, acetone/chloroform and mercaptoethanol. Each sample was run for 48 hours using Soxhlet’s apparatus extraction. The test organisms were K. pneumoniae, P.aeruginosa, E. aerogenes, S. aureus and E. coli. Agar well diffusion method was used to evaluate and compare the antibacterial activity in samples. Amikacin was used as the control to inhibit overgrowth of the bacteria. Among these six extracts, the acetone/chloroform extract of fresh sample showed greater activity against all test organisms. Acetic acid also proved next level of inhibition. Ethanol extract of fresh sample showed moderate activity except E.coli. Mercaptoethanol showed minimum activity in all test organisms. In dry sample acetone/chloroform extracts showed greater activity against three bacterial strains and minimum activity against K. pneumoniae and P. aeruginosa. Acetic acid also performed well. Mercaptoethanol extract of dry sample showed lesser activity and these extracts did not inhibit some bacterial strains. Our current study confirmed that acetone/chloroform and at the next line acetic acid and ethanol extracts reported greater antimicrobial activity against harmful pathogenic test bacteria.
Keywords: Harmful Antimicrobial; Bioactivity; Drug; Extraction and Marine
The oceans occupy more than 70 percentage of the earth. It is endowed with rich natural resources for countless organisms which have significantly contributed to economic and research development. In view of the fact that marine organisms live in complex habitats and are exposed to extreme conditions such as salinity, pressure, temperature fluctuations; they produce a wide variety of new secondary metabolites. The marine life constitutes 80% of overall world biota. Quite a lot of microorganisms are still unexploited in the sea water and sediment. They have special abilities to produce antioxidants, antimicrobials, metabolites with anticancer, anti-inflammatory and anti-diabetic functions (Chairs, et al. [1]). During last decades there has been an increase in research on marine crustaceans, molluscs, echinoderms with particular interest on their secondary metabolites with desirable antimicrobial properties. More or less 10,000 pharmacological bioactive compounds have been derived from marine invertebrates. The marine compounds may possess biological activities such as antihelminthic, anticoagulant, antiplatelet, antimalarial and antituberculosis properties. Some of the biomolecules stated are structurally unique while others belong to known classes of compounds, peptides and proteins (Garcia, et al. [2]). The microbial pathogens are associated with various skin infections, urinary tract infections, gastro intestinal infections and water bone diseases. Due to antimicrobial actions some of the biologically active natural marine organisms have become excellent sources of new and effective antifungal, antimicrobial, antiviral and anti-inflammatory drugs. At the present time increasing resistance to antibiotics by several pathogens made the discoverers to develop marine extracts and drugs to cure infectious diseases. Emergence of resistance of pathogenic microorganisms to majority of antibiotics has enhanced morbidity and mortality rate. Clinical and health problems that arise from antibiotic resistance and multi resistance bacteria became harder and eventually impossible to treat. Consequently this triggered the search for new drugs. In the present days the health sector often uses synthetic drugs for the treatment for these diseases (Dhinakaran, et al. [3]).
Microorganisms have developed increasing resistance of pathogens to antibiotics; there is a public health priority for exploring and developing cheaper and effective natural antimicrobial agents with better potential, fewer side effects than antibiotics, good bioavailability and minimal toxicity (Karthikeyan, et al. [4]). Besides these, the effective use of conventional drugs available in markets has been severely affected due to the increasing incidence and development of multiple resistance mechanism in microbes. Hence there is always a continuous need for search to find new drugs with novel mechanism of action to compact these problems. It is marvellous that the nature has gifted the basic and wonderful source for invention of new and innovative drugs from plants, animals and microorganisms which has exhibited striking structural diversity in generating secondary metabolites (Boujaber, et al. [5]). Marine natural products have drawn the attention of researchers in the recent years due to their pharmacological value. Cuttlebones are the unexploited source for antibacterial activity in some dangerous bacteria. In ancient times some naturally available products were used to treat various diseases. Their antimicrobial properties make the pharmacists to add them as basic source in many drugs. Cuttlebones seem to play a positive role in the control or prevention of some diseases. Due to the antimicrobial action of the biologically active natural products; they have become excellent sources of new and effective drugs. One such investigation was done by (Priya Senan [6]) which showcased maximum activity of Cuttlefish Sepia pharaonis against human pathogens. (Nithiya, et al. [7]) studied the ink extracts of cuttle bone showed the anti-bacterial activity against some human pathogens. (Vasantharaj, et al. [8]) reported the methanolic extracts from the ink of Sepia sp. demonstrated antibacterial activity. The methanolic extracts had inhibitory activity especially against grampositive bacteria E.coli, Proteus vulgaris and Pseudomonas aeruginosa.
(Ramaswamy, et al. [9]) reported the antibacterial activity of a polysaccharide isolated from cuttlebone of Sepia aculeate and S. brevimana and methanolic extracts of the body tissue of Sepia parshadi. In this study methanolic extracts illustrated maximum activity against human pathogens. (Fadjar, et al. [10]) studied the raw ink extracts of cephalopods which can be used as antibacterial activity with a highly significant effect. (Yuvaraj, et al. [11]) tested antibacterial activity on extracts from cuttlefish ink, the result of which declared that the extract had inhibitory activity against several bacteria such as E. coli, P. aeruginosa, S. epidermidis and Klebsiella pnemoniae. (Du-Tie-Ping, et al. [12]) studied the antibacterial activity of extracts from ink sac of cuttle fish on bacteria Staphylococcus aureus and E.coli. (Sherief, et al. [13]) isolated, purified and characterized the antimicrobial and anticancer agents from the accessory nidamental gland and ink gland of the cuttle fish Sepia pharaonis. (Shanmugam, et al. [14]) reported the crude and purified sample of Glycosaminoglycans (GAGs) from cuttle fish (Euprymna berryi) showed activity against five pathogenic bacteria. The maximum antibacterial activity was showed in Shigella sp.
The minimum antibacterial activity demonstrated against E.coli. (Shanmugam, et al. [15]) reported the cuttle bone extracts of S. aculeate and S. brevimana showed antibacterial activity against almost 9 pathogenic bacterial strains viz B. subtilis, E. coli, K. pneumonia, S. aeureus, V. cholerae, S. thyphi, P. aeroginosa, Shigella sp, V. parahaemolythicus. The activity was recorded in almost all the concentrations except in negative control. (Anand, et al. [16]) reported antibacterial activity from the extracts of various cephalopods. The highest activity of gram-positive bacteria indicated by Sepia ink extracts. (Kicklighter, et al. [17]) studied the methanolic extracts of cephalopods showed maximum activity against human pathogens. The result obtained that Aeromonas hydriphilla were effectively inhibited by the ink extracts of cuttle fish. Approximately 16,000 marine natural products that are used in antimicrobial agents for the treatment of bacterial infection. Even though there are many reports in antibacterial activities and their proximate composition available in view of many organisms, peculiar parts and their products study were limited. Again certain studies illustrated that cuttlebone collected from the invertebrate “cuttle fish” have unexploited metabolites. In order to unravel the antibacterial activity, five selected pathogenic bacterial strains of bacteria in cuttle bone extracts were tested for further evaluation of its immune efficiency and its biological activity.
Collection of Sample
Cuttlebone is also known as cuttlefish bone. It is a hard, brittle internal structure found in all Sepidae family. Generally, the genus which belongs to sepidae is commonly known as cuttlefish. The cuttlebone samples were collected from Leepuram coastal area, which is located in Kanyakumari, Tamilnadu, India. For the present study, two types of samples such as dried and fresh cuttlebones were collected.
Description of Sample
Cuttle bone is the calcareous internal shell of the cuttlefish. Cuttle fish has 8 arms and 2 tentacles and are found in oceans all over the world. Cuttlebone is composed primarily of aragonite. It is chambered, gas filled shell used for buoyancy control. Siphuncle is highly modified and is on the ventral side of the shell. The microscopic structures of cuttlebone consist of narrow layers connected by numerous upright pillars. The largest cuttlefish belongs to Sepia apama, the giant Australian cuttlefish. It lives between the surface and maximum depth of 100 meters, and it reaches the maximum size of 50cm. The cuttlebone is used to cure hyperuricemia, gastric ulcer bleeding. It is also used to feed birds because the cuttlebone contains high calcium content (Priya Senan [6]). The major component of cuttlebone is 85 percent of calcium carbonate. The second leading component is organic material which makes up to 8.9 percent of carbohydrate material. This calcium builds strong bones and it is also vital dietary supplement for birds with necessary minerals and calcium. These components are used for bone formation and blood clotting in birds. It is the natural product, and it does not contain toxins or contaminants. Because of the salty and warm properties, cuttle fish bone is used as traditional medicine. Internally it is taken to stop bleeding of uterus. Tropically, cuttle fish bone can be used as poultice to skin rashes, ulcers and lesions (Nithiya et al. 2011).
Preparation of Extracts
The samples are clearly washed with distilled water and allowed to air dry. The samples were powdered separately. 20gm of each dry and fresh sample was extracted using ethanol, methanol, acetic acid, chloroform, acetone mixture and water for 48h. Then the extracts were dried in a hot air oven (Manilal, et al, [18]).
Preparation of Nutrient Broth
13g of nutrient agar powder is mixed with 1L distilled water. This nutrient agar powder is dissolved completely in distilled water. Then the medium was poured into the conical flask and sterilized by a dry autoclave at 1210C for 15 minutes (Ravikumar, et al. [19]).
Cuttlebone Extraction
Cuttlebone was extracted by using soxhlet apparatus (Grace, et al. [20]). 20gm of cuttlebone powder was filled into the porous cellulose thimble. 200ml of solvent was added to the round bottom flask attached to soxhlet extractor. The cuttlebone powder was loaded into the thimble which was placed inside the soxhlet extractor. The arm is lagged with glass wool. The solvent was heated by setting a fixed temperature. With the help of heating mantle, solvent was evaporated through the apparatus of the condenser. Condensate was dripped into the reservoir containing the thimble. Once the level of solvent reaches the siphon it comeback into the flask and the cycle was repeated. By this way the whole process was run for 48h. Different extraction solvents and conditions are given in (Table 1).
Antimicrobial Activity of Cuttlebone Extracts
Test Organisms: The pathogenic bacteria such as Klebsiella pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Enterobacter aerogenes were used to check their antibacterial activity.
1.Klebsiella Pneumoniae
K.pneumoniae is a gramnegative, encapsulated, non-motile bacterium that is found in the normal environment. It was associated with pneumonia in the alcoholic and diabetic patient population. K. pneumoniae is a well known cause of community acquired pneumonia. The bacterium typically colonizes human mucosal surfaces of the oropharynx and gastrointestinal tract. K. pneumonia is also present in the respiratory tract and faeces of about 5% of normal individuals. It produces extensive hemorrhagic necrotic consolidation of the lung. It also produces urinary tract infection and bacteraemia with focal lesions in debilitated patients (Srinivasan, et al. [21]).
2.Escherichia Coli
E.coli is a gram negative, facultative anaerobic, rod shaped coliform bacteria. It is commonly found in the lower intestine of warm blooded organisms. Most of the E. coli strains are harmless, but some serotypes can cause serious food poisoning in their hosts which are occasionally responsible for food contamination. Some types can cause illness in humans including diarrhoea, abdominal pain, vomiting and fever. Some other types of E. coli infection can lead to urinary tract infection, respiratory illness, pneumonia and other illness like meningitis.
3.Staphylococcus Aureus
S. aureus is a grampositive bacterium which is spherical in shape and forms grape like cluster. It is a facultative anaerobic organisms (capable of growth both aerobically and anaerobically). It commonly causes skin infection including abscesses, respiratory infection such as sinusitis and food poisoning. Pathogenic strains often promote infection by producing virulence factors such as potent protein toxins and the expression of cell surface protein that binds and inactivates antibodies.
4.Pseudomonas Aeruginosa
P. aeruginosa is an aerobic, motile, gramnegative rod shaped bacteria. It also causes chronic pneumonia in cystic fibrosis patients and wound infection with green exudates in burn patients. It also causes diseases in plants and animals including humans. It is ubiquitous in soil and water, but occurs regularly on the surfaces of plants and occasionally on the surfaces of animals. Other infection caused by Pseudomonas species includes endocarditis, pneumonia, infections of the urinary tract and their wounds.
5.Enterobacter Aerogenes
E. aerogenes is a gramnegative, rod shaped with round ended bacterium. It lives in both aerobic and anaerobic environment. It causes respiratory infection and urinary tract infection. It is found in soil, water and dairy products and also lives in intestines of animals as well as humans. They are most frequently found in the gastrointestinal tract and are studied in clinical sites in stool samples.
Sub Culturing of Bacterial Strains
The bacterial strains were sub cultured in nutrient agar medium (g/l) (peptic digest of animal tissue-5, sodium chloride-5, beef extract1.5, yeast extract -1.5 and agar 15) was used for bacterial strains. Later all cultures were maintained at 4oC for further studies.
Antibacterial Assay
Antibacterial activity of solvent extracts was determined by agar well diffusion method. Each bacterial culture was spread on nutrient agar plates with sterile swab moistened with the bacterial suspension. 6mm diameter wells were punched into agar medium and 20μl of cuttlebone extracts were loaded into the well. Amikacin (10μl) was used as the positive control and DMSO was used as a negative control. All plates were incubated for 24h at 37oC. The diameter of the zone of inhibition was measured in millimetre (mm).
Antibacterial Activity of Cuttlebone Samples
The present study carried out on the cuttlebone (fresh and dry) samples was to evaluate the antibacterial activity using various extracts. Cuttlebone is extracted by using water, ethanol, methanol, acetic acid; acetone/chloroform and mercaptoethanol. Different extracts were tested for their antibacterial activity against pathogenic bacteria (Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Enterobacter aerogenes). All extracts were tested for their antibacterial activity. The cuttlebone extracts with different solvents showed antibacterial activity against both grampositive and gramnegative bacteria. The acetone/chloroform extracts of cuttlebone showed highest inhibition zone. Overall the acetone/chloroform extracts of fresh cuttlebone sample showed highest inhibition of all microorganisms compared to all other extracts.
Antibacterial Activity of Fresh Cuttlebone Samples Using Different Solvents: The results obtained in fresh samples with different extracts were interpreted in (Table 2, Figure 1, Picture 2-4). The water extract showed the 7mm inhibition zone against Klebsiella pneumonia, Pseudomonas aeruginosa (8mm), Enterobacter aerogenes (9mm), Staphylococcus aureus (8mm), Escherichia coli (no activity). Ethanol extract showed the inhibition zone of pneumoniae (12mm), P. aeruginosa (10mm), E.aerogenes (11mm), S.aureus (10mm) and E.coli was having no activity. Methanol extract showed inhibition zone of K. pneumoniae (8mm), P. aeruginosa (7mm), E. aerogenes (8mm), S.aureus (7mm) and E.coli (no activity). Acetone/chloroform extract showed inhibition zone of K. pneumoniae (23mm), P. aeruginosa (20mm), E. aerogenes (19mm), S.aureus (13mm) and E. coli (18mm). Acetic acid extract showed inhibition zone of K. pneumoniae (15mm), P. aeruginosa (11mm), E.aerogenes (12mm), S.aureus (9mm) and E.coli (8mm). Mercaptoethanol extract showed inhibition zone of K.pneumoniae (7mm), P. aeruginosa (8mm), E. aerogenes (7mm), S. aureus (7mm) and E. coli (8mm).
Figure 2 Figure 1: Antibacterial activity of fresh cuttlebone sample in different solvents. Acetic acid extract showed inhibition zone of K. pneumoniae (15mm), P. aeruginosa( 11mm), E.aerogenes(12mm), S.aureus(9mm) and E.coli (8mm). Mercaptoethanol extract showed inhibition zone of K.pneumoniae (7mm),P. aeruginosa(8mm), E. aerogenes (7mm), S. aureus(7mm) and E. coli (8mm).
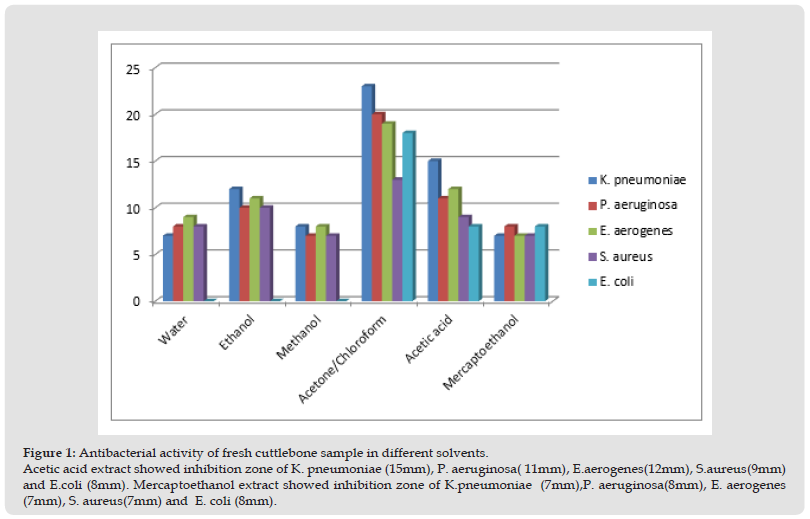
Figure 5 Picture 4: showing zone of inhibition of fresh cuttlebone sample using Acetone/ Chloroform, Acetic acid and Mercaptoethanol extracts.
Antibacterial Activity of Dry Cuttlebone Samples Using Different Solvents: The dry cuttlebone samples were extracted by using water, ethanol, methanol, acetone/chloroform, acetic acid and mercaptoethanol (Table 3, Figure 2, Picture 2, 3 & 5). The water extract showed the inhibition zone of pneumoniae (7mm), P. aeruginosa (7mm), E. aerogenes (8mm), S. aureus (7mm), E. coli (7mm). Ethanol extract showed the inhibition zone of K. pneumoniae (12mm), P. aeruginosa (10mm), E. aerogenes (10mm), S. aureus (10mm) and found no activity in E.coli . Methanol extract showed inhibition zone of K. pneumoniae (7mm), P. aeruginosa (8mm), E. aerogenes (8mm), S. aureus (7mm) and E. coli (8mm). Acetone/chloroform extract showed inhibition zone of K. pneumoniae (9mm), P. aeruginosa (9mm), E. aerogenes (15mm), S. aureus (12mm) and E. coli (13mm). Acetic acid extract showed activity in K. pneumoniae(10mm), P. aeruginosa(11mm); inhibition zones of E. aerogenes (10mm), S. aureus (7mm) and E. coli (7mm). Mercaptoethanol extract showed inhibition zone of K. pneumoniae (7mm), E. aerogenes (7mm), no activity was noticed in P. aeruginosa, S. aureus and E. coli.
Antibacterial activity is completely associated with the compounds that kill bacteria or slow down their rate of growth without being extensively toxic to nearby tissues. Most recently discovered antibacterial agents are modified natural compounds and this modification is done through chemical mode. Effective treatment of disease causes the development of new pharmaceuticals or some potential sources of novel drugs. Commonly available medicinal plants are the excellent sources of drugs to cure ailment troubles. Some other marine products are promising to inhibit some dangerous bacteria. A vast number of natural products have been recognized as valuable resource of natural antibacterial agents as an alternative that can potentially be effective in treatment of these problematic bacterial infections. In our present study similar idea gave good effects on the selected test organisms. The current study was framed to evaluate and compare the antimicrobial activity by utilizing locally available cuttlebones present in the coastal area. Samples were extracted by using different solvents. As evident from two samples, they were utilized to study the ability of growth inhibition of pathogenic bacteria (Tables 2 & 3). These two samples were separately extracted by using water, ethanol, methanol, acetone/chloroform, acetic acid, mercaptoethanol. The bacterial strains were K. pneumonia, E. coli, E. aerogenes, S. aureus and P. aeruginosa.
During fresh sample extraction acetone/chloroform extract showed maximum zone of inhibition against five test organisms. Next to acetone chloroform mixture Acetic acid proved to have good antibacterial activity Ethanol extract showed moderate zone of inhibition in four bacterial strains except E. coli. Ethanol extract did not inhibit the growth of E. coli bacteria. Mercaptoethanol extract showed minimum zone of inhibition in all test organisms. Water extract showed minimum inhibition zone in four bacterial strains. E. coli bacteria in water were found to have no activity (Figures 1 & 2). Dry sample also showed maximum zone of inhibition in acetone/chloroform extract against three bacterial strains except K. pneumoniae and P. aeruginosa. These two bacterial strains showed minimum inhibition zone. Ethanol extract showed moderate zone of inhibition in four bacterial strains. But E. coli was not inhibited by ethanol extract. Water and methanol extracts showed minimum zone in all test organisms. Acetic acid extract proved moderate results in test bacterial strains. Mercaptoethanol showed minimum zones of inhibition in two bacterial stains and others like P. aeruginosa, S. aureus, E. coli) observed to have no activity.
When Comparing these two samples fresh sample showed maximum zones of inhibition in acetone/chloroform extract than dry sample. Next to this acetic acid proved to be a better extract. Not only had the acetone/chloroform extract, all other extracts of fresh sample showed more antibacterial activity against test organisms except E. coli. In water, ethanol and methanol extract did not showed any activity against E. coli. However, fresh sample was proved to be a superior one than dry samples. Similar study carried out by (Satheesh Kumar, et al. [22]) proved antimicrobial activity against Stevia rebaudiana of four solvent extracts (ethyl acetate, acetone, chloroform, water) against 6 bacteria by using agar well diffusion method. In this study acetone extract showed greater activity against gram positive and negative bacteria. (Agarry, et al. [23]) compared antibacterial activity of gel and leaf of Aloe vera. In this study he reported the plant leaf extracts showed positive result in K. pneumoniae, S. aureus, E. coli. (Kowsalya, et al. [24]) reported the antimicrobial activity of two seaweed species. These two seaweeds are extracted by ethanol, chloroform, methanol and water. Antimicrobial activity indicated that the active constituents in the extraction of marine algae which could be exploited for the production of innovative drugs. Present investigation was also in conformity with these study reports.
(Tadhani, et al. [25]) recorded very low activity for water extract of Stevia rebaudiana leaves. Water extracts do not have activity against bacteria. Similar findings were proved with our water extraction. (Barat, et al. [26]) for EDTA extract (polysaccharide) of Dorythuthis sibogae gladiolus recorded 10mm inhibition in E. coli and K. pneumoniae and 7mm inhibition in S.aureus. (Pushparaj, et al. [27]) reported antibacterial activity of seaweeds against six human pathogenic bacteria. Best antibacterial activity was recorded in ethanol extracts. Our results proved that ethanol was moderately good. (Ahmed, et al. [28]) reported the antimicrobial activity of the extracts and their potency was quantitatively assessed by the presence and absence of inhibition zone and zone diameter (Ashraf Mostaf, et al. [29-32]). Only alcoholic extracts was found to be a better solvent for extraction of active antimicrobial substances compared to water and hexane. Cuttlebone samples of the present study showed antibacterial activity against both gram positive and negative bacteria. The result recommended the cuttlebone can produce antibacterial substances instantly to combat bacterial infection. Compared to these studies, antibacterial activity of acetone/chloroform extract of cuttlebone was a superior one and the second protection was given by acetic acid and ethanol proved to be a moderate one.


